Article Text
Abstract
Introduction In critically ill children, pain management is complex owing to cognitive development and the nature of hospitalisation in paediatric intensive therapy units. Although there are many protocols and guidelines for pain control via pharmacological interventions, non-pharmacological practices should also be explored and disseminated for their potential benefit.
Methods and analysis A systematic literature search will be performed using the following databases: Academic Search Premier, Cumulative Index to Nursing and Allied Health Literature, Cochrane Library, Excerpta Medica Database, Virtual Health Library, Medical Literature Analysis and Retrieval System Online, ScienceDirect, Scopus, Web of Science Core Collection, Theses from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Dart Europe, Open Access Theses and Dissertations and grey literature from Google Scholar. The research will consider quantitative and qualitative studies, mixed-method studies, systematic reviews, text articles, opinion articles, letters to editors and editorials in any language and from any database. The following will be eligible for inclusion: (1) newborns, infants, children and adolescents; and (2) non-pharmacological therapies used for pain in paediatric intensive care.
Ethics and dissemination This study does not require ethical approval. The results of this research will be disseminated through social media channels and podcasts about pain in children.
Trial registration number This protocol has been registered with the Open Science Framework (DOI 10.17605/OSF.IO/DZHKT).
- Paediatric anaesthesia
- Paediatric intensive & critical care
- PAIN MANAGEMENT
- COMPLEMENTARY MEDICINE
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Promotes updates on what is known about non-pharmacological therapies.
Presents gaps in knowledge that still need to be addressed.
Enables the selection of studies in other languages.
A modification and downsizing occurred within the initial reviewer’s team, potentially introducing bias to the evidence.
Introduction
Admission to the paediatric intensive care unit (PICU) exposes paediatric patients to various pain experiences, and approximately 45%–72% of these patients experience pain daily either due to their critical illness, procedures, therapies or surgeries.1 As a result, critically ill children have more experience with intense pain, as they are subjected to more painful procedures than children in other hospital divisions, such as medical and surgical units.2 In the PICU, pain can be caused by the underlying illness or injury, complications of the primary disease, frequent medical procedures that result in pain (eg, incisions, wound care and injections), and supporting and monitoring systems (eg, suctioning an endotracheal tube, manipulation or stripping of drains, removal of catheters or drains).2 3 Tissue hypoxia that develops due to low oxygen saturation, cardiac output or anaemia can also cause pain2 3; prolonged immobilisation can result in pain in the joints and pressure points and from changing positions.2 3
Despite recent scientific and technological developments, paediatric patients frequently lack adequate pain relief.2–9
Moreover, lack of pain relief is considered one of the most commonly reported adverse events in the US PICUs.2 According to the International Association for the Study of Pain, pain relief has been discussed as a human right by international institutions since 2004.10 However, treating pain in children remains challenging. One of the obstacles to the management of pain control in paediatrics is the heterogeneity of pain perception and response among different paediatric age groups.1 10 11
Although pain diagnosis is often performed using self-reporting scales, this method has limitations in paediatrics. For example, neonates and young children may not have yet achieved the developmental level necessary for effective verbal communication. In this group, the scales use behavioural observation and physiological measures. Moreover, parents and caregivers have more accurate observations than healthcare providers in identifying pain-related facial expressions and responses in children.1 In The Pain, Agitation, Neuromuscular Blockade, and Delirium in Critically Ill Paediatric Patients with Consideration of the PICU Environment and Early Mobility (PANDEM) Guidelines, Smith et al discussed that the pain assessment tools could be classified as self-report or observation scales.12 Self-report scales are considered the gold standard and have been validated in children over 3 years of age, although self-assessment from the age of 6 years was considered more reliable. The most used paediatric self-report scales are the Analogue Visual Scale, Numerical Classification Scale, Oucher Pain Scale and Wong-Baker Pain Scale. Alternatively, observation scales incorporate behavioural aspects associated with physiological variables to evaluate the pain in children who are unable to self-report the pain. Among these tools, the Face, Legs, Activity, Cry, Consolability, Comfort and Comfort-B scales are the most commonly used observation tools in critically ill children.12
Measuring and treating pain in critically ill children represent a significant endeavour for health professionals,1 and the risk factors for experiencing pain vary from specific factors in paediatric patients to PICU-related factors.12 The perception of pain may be influenced by age, anxiety, fear, comorbidities, concern about family separation, strange environments, barriers to verbal communication and racial bias. Indeed, another factor that can change the way children feel and show their pain is cultural and social differences.1 The PICU-related factors involve mechanical ventilation, invasive procedures, invasive devices, the use of multiple medications, frequent sleep interruptions and reduced mobility.12
The proper administration of analgesia contributes to pain relief, improves psychomotor agitation, facilitates the maintenance of invasive devices, optimises synchronisation between the mechanical ventilator and child, and decreases oxygen consumption and stress response. The decrease in these events is related to proper pain management in PICUs.12 Pain management in children requires pharmacological and non-pharmacological therapies,13 with pharmacological interventions typically involving protocols of opioids alone or with other non-opioid drugs.12 As the use of pain medications is related to side effects and misuse of opioids, non-pharmacological interventions have been explored by professionals and researchers. Although there is a consensus that combining both approaches is more effective, the amount of information on non-pharmacological pain treatment in critically ill children is limited.13
Non-pharmacological interventions can be categorised as behavioural, cognitive, restorative and complementary therapies.14 Interventions, such as oral sweet solution, non-nutritive sucking, positioning, skin-to-skin contact and modifying environmental stimuli,15 16 have the potential to alleviate stress generated by hospitalisation, improve quality of life and prevent changes in the physiology and behaviour of neonates.15 Non-pharmacological therapies may also help improve the effectiveness of medications or even contribute to reducing their use, thus improving the scores related to adverse events of drug use. A previous medical record analysis enabled an observational cohort study in 15 PICUs17; the most used measures were repositioning, decreasing environmental stimuli, carer presence, distraction and music therapy.13 17 Additionally, Yaz and Atay conducted a transverse study to describe the nurses’ use of non-pharmacological methods in paediatric intensive care clinics during the COVID-19 pandemic.18 While the pandemic changed the time and training available for healthcare staff interested in this area, the alternatives to pharmacotherapy commonly used by nurses in paediatric interventions remain the same and include embracing, massage, pacifier use, therapeutic touch, toy distraction, musical therapy and speaking, providing preprocess information, heat/cold application, parent involvement, kangaroo care, giving sucrose, video distraction, postapplication rewarding, breathing exercise and dreaming.18
Other forms of non-pharmacological therapies involve integration with medication to decrease environmental stressors and facilitate relaxation, distraction and sleep.12 A scoping review held in 2019 mapped pain management in PICUs. These interventions involved guided imagery; hypnosis; detailed inquiry, including interview technique that rescues information on thoughts and feelings related to pain; parental presence; distraction; a combination of psychological, physical and pharmacological interventions, such as positioning, guided imagery, hypnosis and parental education; acupuncture; stroking and soothing, holding and rocking; and environmental modifications, such as a quiet environment, dim lights, limiting visitors to decrease noise and music.19
Despite advancements in pain management in PICUs, various methodologies and guidelines advocate the development of further research on this topic. Ismail et al reported that all the articles identified from the literature search were published in English and that the studies focused only on quantitative designs.19 Recently, the PANDEM guidelines12 suggested that research must be conducted to validate the information on the impact of acupuncture on postoperative or procedural pain. As a contribution to the knowledge of non-pharmacological therapies, this scoping review aims to add data collected from other study designs and languages to explore new evidence on pain management in critically ill children with a focus on acupuncture techniques. In addition, it addresses aspects related to the different ages of children in PICUs who are receiving non-pharmacological treatment for pain. Furthermore, paediatric intensive care professionals need access to the best and most up-to-date scientific evidence on non-pharmacological therapies for pain management in PICUs.6 8 9 The development of a quality scoping review can provide data that explores the phenomenon of non-pharmacological therapies in critically ill children and helps interpret the various factors involved in paediatric critical care. Thus, this review aims to map the research that used non-pharmacological therapies for pain management in PICUs and provide information to investigate the characteristics of these techniques.
Methods and analysis
Scoping reviews aim to synthesise evidence for researchers, clinicians and policy-makers, gather and describe the evidence, and present an easily illustrative summary.20 As non-pharmacological therapies exhibit a multiplicity of techniques with varied health impacts and use according to different patient ages, the scoping review was deemed the most appropriate review to illustrate the different nuances of this topic in the paediatric spectrum, which is also heterogeneous.
This scoping review will be conducted according to the chapter on scoping reviews in the Joanna Briggs Institute (JBI) Manual for Evidence Synthesis21 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (online supplemental appendix 1).22 This protocol is registered in the Open Science Framework (https://doi-org.ezproxy.u-pec.fr/10.17605/OSF.IO/GKETX). This review will be conducted in five phases: (1) identification of the research question; (2) identification of studies; (3) selection of studies; (4) mapping and comparison of the data; (5) collecting, summarising and reporting the results23; (6) consultation with stakeholders about the results (optional).24
Supplemental material
Patient and public involvement
This study does not involve patients or the public.
Identifying the research question
The research question for this scoping review is ‘What non-pharmacological therapies have been used for pain management in PICUs?’ To support this query, four subquestions were developed:
What techniques were used among different age groups?
What scores on the pain scales define the use of non-pharmacological therapy?
How were non-pharmacological therapies used to reduce pain in the PICU?
What therapies impacted decreased pain, mechanical ventilation duration or reduction in length of stay in the PICU?
What factors assess the effectiveness of non-pharmacological interventions?
To construct the research question, we used the population, concept, context strategy recommended by the JBI20 and the table proposed by Ahmad et al (table 1).25
The inclusion and exclusion criteria are based on the population, concept, context framework
This review will consider studies that explore unconventional pain management therapies. In healthcare, complementary therapies indicate the integration of an alternative approach with conventional medicine. If an unconventional health intervention is used instead of traditional medicine, we call it ‘alternative’ therapy. If an intervention occurs through the coordinated action of traditional medicine and an unconventional approach, the term ‘integrative’ therapy is used.26
However, identifying what therapies constitute complementary, alternative and integrative medicine is complex.27 One operational definition of ‘complementary and alternative medicine’ was proposed by Cochrane researchers in 2011. There was also a discussion on integrative health and integrative medicine that influenced the National Center for Complementary and Alternative Medicine in the USA and changed it to the National Center for Complementary and Integrative Health (NIH).26 28
This review will adopt the term non-pharmacological therapies; although it is not described in the U.S. National Library of Medicine Medical Subject Headings (MeSH) terms, it incorporates complementary therapies, alternative therapies, integrative therapies and integrative medicine.
Identifying relevant studies
Our search strategy was developed in collaboration with a librarian at a leading university in Brazil and aims to locate primary published studies, reviews and text articles. First, a limited initial search will be performed on the Cumulative Index of Nursing and Allied Health Literature (CINAHL) and Medical Literature Analysis and Retrieval System Online (MEDLINE) to identify relevant terms and keywords to develop the final search strategy. For transparency and replication of the review, a chart was created based on the protocol by Ahmad et al25 and Luberenga et al,29 which outlines the details of the strategy with MeSH terms and text words (box 1).
Medical subject headings (MeSH) terms/text words
Population
Newborns, infants, children and adolescents
Concept
"pain management" [MeSH Terms] OR "pain management" [Text Word] OR "pain relief" [Text Word] OR "pain control" [Text Word] OR "pain reduction" [Text Word] OR "managing pain" [Text Word] OR "analgesia" [MeSH Terms] OR "analgesia" [Text Word]
"pain measurement" [MeSH Terms] OR "pain assessment" [Text Word] OR "pain measurement" [MeSH Terms] OR "pain scale" [Text Word] OR "pain tool" [Text Word] OR "pain assessment tool "[Text Word] OR "pain instrument" [Text Word] OR "pain intervention" [Text Word] OR "pain measurement" [MeSH Terms] OR "pain measurement" [Text Word]
1 OR 2
"integrative medicine" [MeSH Terms] OR "integrative medicine" [Text Word] OR "complementary therapies "[MeSH Terms] OR "complementary medicine" [Text Word] OR "complementary therapies" [MeSH Terms] OR "alternative medicine" [Text Word] OR "integrative therapy" [Text Word] OR "integrative therapies" [Text Word] OR "complementary therapy" [Text Word] OR "complementary therapies" [MeSH Terms] OR "complementary therapies" [Text Word] OR "alternative therapy" [Text Word] OR "complementary therapies" [MeSH Terms] OR "alternative therapies" [Text Word] OR "alternative treatment" [Text Word]
"non-pharmacological intervention" [Text Word] OR "non-pharmacological interventions" [Text Word] OR "non-pharmacological therapy" [Text Word] OR "non-pharmacological therapies" [Text Word] OR "non-pharmacological treatment" [Text Word]
"aromatherapy" [MeSH Terms] OR "aromatherapy" [Text Word] OR "oils, volatile" [MeSH Terms] OR "essential oils" [Text Word] OR "aromatherapy" [MeSH Terms] OR "aroma therapy" [Text Word]
"acupuncture" [MeSH Terms] OR "acupuncture therapy" [MeSH Terms] OR "acupuncture" [Text Word] OR "acupuncture therapy" [MeSH Terms] OR "acupuncture therapy" [Text Word] OR "acupuncture treatment" [Text Word] OR "acupuncture, ear" [MeSH Terms] OR "acupuncture ear" [Text Word]
"mind-body techniques" [Text Word] OR "mind-body therapies" [MeSH Terms] OR "mind-body therapies" [Text Word]
"breathing techniques" [Text Word] OR "breathing exercises" [MeSH Terms] OR "breathing exercise" [Text Word]
"guided imagery intervention" [Text Word] OR "guided imagery interventions" [Text Word] OR "imagery, psychotherapy" [MeSH Terms] OR "guided imagery" [Text Word] OR "guided relaxation" [Text Word]
"hypnosis" [MeSH Terms] OR "hypnosis" [Text Word] OR "hypnotherapy" [Text Word]
"biofeedback, psychology" [MeSH Terms] OR "biofeedback" [Text Word] OR "biofeedback therapy" [Text Word]
"music therapy" [MeSH Terms] OR "music therapy" [Text Word] OR "music intervention" [Text Word] OR "musical therapy" [Text Word] OR "music-based intervention" [Text Word] OR "therapeutic music" [Text Word]
"progressive muscle relaxation" [Text Word] OR "muscle relaxation" [MeSH Terms] OR "muscle relaxation" [Text Word]
"sleep therapy" [Text Word] OR "sleep" [MeSH Terms] OR "sleep" [Text Word]
"massage therapy" [Text Word] OR "massage" [Text Word] OR "massage therapies" [Text Word]
"physical therapy modalities" [MeSH Terms] OR "physical therapy" [Text Word] OR "physical therapy modalities" [MeSH Terms] OR "physiotherapy" [Text Word]
4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17
Context
"paediatric intensive care unit" [Text Word] OR "picu" [Text Word] OR "paediatric critical care unit" [Text Word] OR "paediatric critical care" [Text Word] OR "intensive care units, paediatric" [MeSH Terms]
3 AND 18 AND 19
The keywords in the titles and abstracts of articles and the indexing terms used in the articles will be used to guide a complete search strategy for Academic Search Premier, CINAHL, Cochrane Library, Excerpta Medica Database (Embase), Virtual Health Library (VHL), MEDLINE, Science-Direct, Scopus and Web of Science Core Collection to be disclosed with the results of the scoping review.
The search strategy will consider the particularity of each information source, including all the identified keywords and indexing terms. The strategy will consider articles published in any language and with any database at the start or date of insertion. We use independent professional translation services for the authors’ translation of articles in non-native languages.
In addition to the electronic databases, contact will be made with the study authors, when necessary, to clarify any doubts. This protocol will consider sources of unpublished studies: Theses from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Dart Europe (DART-E), Open Access Theses and Dissertations (OATD) and grey literature in Google Scholar. The online supplemental appendix 2 provides a list of possible search queries.
Supplemental material
Study selection
The search process will be carried out in two stages: (1) reading the title and abstract (first set of records); and (2) reading the full article (second set of records). Screening, which will result in the first set of records, will be performed by two pairs of reviewers who will divide the search as follows: (1) pair A (IGMA and JKdSD) will perform the search using the following sources: Academic Search Premier, CINAHL, VHL, Embase, Science-Direct, DART-E, OATD and grey literature from Google Scholar; (2) pair B (SCMdA and JAT) will perform the search using the following sources: Cochrane Library, MEDLINE, Scopus, Web of Science and CAPES.
The first data record will be grouped and loaded in EndNote V.2.0 (Clarivate Analytics, Pennsylvania, USA), and duplicates will be removed. A pilot test will be carried out on two sources of information, CINAHL and MEDLINE, for evaluation according to the inclusion criteria for the review. The potentially relevant articles will be retrieved in full, comprising the second set of records and their citation details, and will be imported into Rayyan (Rayyan Systems, Cambridge, Massachusetts, USA).
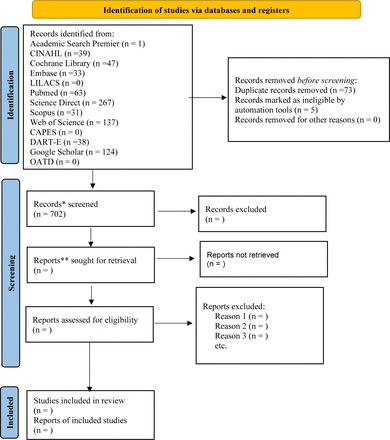
At this stage, any necessary adjustments will be made to the search strategy to meet the inclusion criteria of the review. After completing the pilot test, two reviewers (IGMA and ABdC) will evaluate the full text of the other selected citations to check whether they meet the inclusion criteria. The reasons for excluding full-text articles that do not meet the inclusion criteria will be recorded and reported in the scoping review. A third reviewer (JAT) will resolve the disagreements at any stage of this process. The research results will be fully reported in the final scoping review and presented in a flow diagram (figure 1).30
Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 flow diagram for new systematic reviews which included searches of databases and registers only.30 Research results until July 2023. *Report: a document (paper or electronic) supplying information about a particular study. It could be a journal article, preprint, conference abstract, study register entry, clinical study report, dissertation, unpublished manuscript, government report, or any other document providing relevant information. **Record: the title or abstract (or both) of a report indexed in a database or website (such as the title or abstract for an article indexed in MEDLINE). Records that refer to the same report (such as the same journal article) are ‘duplicates’; however, records that refer to reports that are merely similar (such as a similar abstract submitted to two different conferences) should be considered unique. CAPES, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; CINAHL, Cumulative Index of Nursing and Allied Health Literature; DART-E, Dart Europe; MEDLINE, Medical Literature Analysis and Retrieval System Online; OATD, Open Access Theses and Dissertations.
Mapping and comparison of the data
Data will be extracted using a data extraction tool developed by the authors based on a model proposed by the JBI in table 3.20
Outline of the extraction tool
This tool can then be refined further to address the research question for the scoping review, as required. The feasibility of the extraction tool will be tested on a subset of the second set of records, and this will then be modified and revised for each article included in the data extraction process. Any modifications will be detailed in the full scoping review.
Two reviewers will be involved in data extraction (IGMA and ABdC). Data extraction will occur independently, with cross-checking of the extracted evidence. A third reviewer (JAT) will resolve any disagreements between the authors regarding dissimilarities in terms of data extraction.
Collating, summarising and reporting the results
The total number of studies included will be presented in a summarised table format, using the extraction tool as a guide. Subsequently, the data will be grouped according to the Patterns, Advances, Gaps, Evidence for Practice, and Research Recommendations strategy proposed by Bradbury-Jones and Aveyard.24 Each component represents a domain. The starting point is to produce the ‘Chart of Patterns’. It displays the articles included in the review on one axis and the themes on the other to report the themes expressed in the articles. After the patterns are identified, advances and gaps can be identified and reported, resulting in the last domain of the framework, which is to recommend future research. In addition, the strategy also enables the identification of topics that do not require further research; that is, the scientific community has explored them well.
Ethics and dissemination
As scoping reviews use secondary data from other primary sources, approval for the protocol and review by the Research Ethics Committee will not be necessary. The results of this research will be disseminated through social media channels and podcasts about pain in children.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
We would like to thank Editage (www.editage.com) for the English language and text editing.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors IGMA and JAT contributed to conception and design of this protocol. IGMA, SCMdA, JKdSD, PLOdA and TEdLF contributed to acquisition of data. IGMA conceptualised the research question, and prepared the drafts and manuscript edits. JSAO provided methodological expertise. TEdLF and ABdC helped refine the research question. All authors have contributed to the study design and revised the protocol. All authors have approved the final manuscript.
Funding Rio Grande do Norte Federal University is evaluating this article for funding.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.