Article Text
Abstract
Introduction Diagnosing invasive cutaneous melanoma (CM) can be challenging due to subjectivity in distinguishing equivocal nevi, melanoma in situ and thin CMs. The underlying molecular mechanisms of progression from nevus to melanoma must be better understood. Identifying biomarkers for treatment response, diagnostics and prognostics is crucial. Using biomedical data from biobanks and population-based healthcare data, translational research can improve patient care by implementing evidence-based findings. The BioMEL biobank is a prospective, multicentre, large-scale biomedical database on equivocal nevi and all stages of primary melanoma to metastases. Its purpose is to serve as a translational resource, enabling researchers to uncover objective molecular, genotypic, phenotypic and structural differences in nevi and all stages of melanoma. The main objective is to leverage BioMEL to significantly improve diagnostics, prognostics and therapy outcomes of patients with melanoma.
Methods and analysis The BioMEL biobank contains biological samples, epidemiological information and medical data from adult patients who receive routine care for melanoma. BioMEL is focused on primary and metastatic melanoma, but equivocal pigmented lesions such as clinically atypical nevi and melanoma in situ are also included. BioMEL data are gathered by questionnaires, blood sampling, tumour imaging, tissue sampling, medical records and histopathological reports.
Ethics and dissemination The BioMEL biobank project is approved by the national Swedish Ethical Review Authority (Dnr. 2013/101, 2013/339, 2020/00469, 2021/01432 and 2022/02421-02). The datasets generated are not publicly available due to regulations related to the ethical review authority.
Trial registration number NCT05446155.
- dermatological tumours
- dermatological tumours
- health informatics
- dermatopathology
- diagnostic imaging
- cancer genetics
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- dermatological tumours
- dermatological tumours
- health informatics
- dermatopathology
- diagnostic imaging
- cancer genetics
STRENGTHS AND LIMITATIONS OF THIS STUDY
The BioMEL biobank is population based and includes comprehensive clinical, molecular and image-based data from Swedish melanoma patients.
The BioMEL biobank facilitates translational research on nevi and melanoma biomarkers from blood and tissue biopsies and their microenvironment.
The BioMEL biobank includes unique tissue samples from equivocal nevi and primary melanomas, including very early-stage melanomas (<1 mm tumour depth).
Although carefully selected tissue samples might not represent the whole tumour’s biology, which might bias the results in molecular analyses.
Due to the punch biopsy size (1 mm), included primary lesions are usually ≥5 mm in diameter, which might bias the inclusion of tumours and quota of harvested tumour cells.
Introduction
Invasive cutaneous melanoma (CM) causes most mortalities of the common skin cancer types. In Sweden, CM is the fifth most common cancer type.1 2
An early CM diagnosis is commonly correlated with a thinner melanoma and an earlier stage, which in turn is strongly associated with better disease-specific survival. Hence, methods are warranted to accurately diagnose CM in an early and curable stage. The gold standard for primary melanoma diagnosis is a clinical examination, including dermoscopy, followed by an excisional biopsy and confirming histopathological examination.3 However, diagnosing CM in very early stages is challenging, especially differentiating between equivocal nevi and melanoma in situ. To study tumour progression, all stages of the studied disease are needed. In addition, biological sequences that promote the progression from atypical melanocytes to invasive melanoma remain poorly understood for CM. Unfortunately, current histopathological and dermatological diagnostics are susceptible to subjectivity, as they rely on the experience and expertise of the practitioner.4 Thus, the current reference method for diagnosis, the histopathological examination, is not 100% accurate. Also, surgery always involves risks and possible morbidity for the patient. These facts emphasise the necessity of developing more objective diagnostic techniques to differentiate between benign and malignant melanocytic lesions.5
One advantage of molecular biomarkers is their objectivity, as they can provide quantitative measurements of a disease stage.6 7 Translational research, based on biomarkers and biomedical data from biobanks and population-based healthcare data, is one way to study diagnostics, tumour progression and prognostic factors. A comprehensive biobank established in routine care is well suited for this purpose. The BioMEL biobank is part of regular melanoma care in Southern Sweden. It is a large-scale prospective biomedical data and tissue biobank, harbouring epidemiological and translational data that can serve as a platform for melanoma research. A unique trait of the BioMEL biobank is that tumours ranging from benign but equivocal nevi to invasive melanoma, as well as metastatic melanoma, can be included. At the time of the establishment of the BioMEL biobank, no other biobank had matched blood and tissue samples from patients with nevi and thin melanoma in Europe. This manuscript describes the settings of the BioMEL biobank.
However, several future research objectives, based on data extracted from the BioMEL biobank, can already be listed:
Which are the objective molecular, genotypic, phenotypic and structural differences between nevi and early CM?
Is there a molecular and biologically measurable evolution from equivocal nevi to early CMs?
Can image-based diagnostics of nevi and CM be improved?
Which biomarkers can help in discriminating between dysplastic nevi and early CMs?
Prognosis and treatment response to existing surgical and oncological treatments for metastatic melanoma disease.
Can potential targets for novel treatment alternatives be exposed?
Indeed, BioMEL is already today involved as a supportive platform in more than five ongoing research projects, including two PhD projects. In all these projects, tissue samples (frozen and fresh tissue) from nevi, primary and metastatic melanoma are combined with clinical data and pharmacological treatments. Projects about non-invasively harvested biomarkers are also included in the planned or ongoing projects where BioMEL is the foundation for the research.
Methods and analysis
Infrastructure and predefined diagnoses
BioMEL has been an integral part of standard care for patients exhibiting possible signs of melanoma since 2013. This programme is offered by the dermatology, surgery (including general surgery, ENT (Ear, Nose and Throat), neuro and gynaecology) and oncology departments at Lund, Malmö, Helsingborg and Kristianstad hospitals. Biological samples and health data information are prospectively gathered at the BioMEL biobank. All histopathological diagnoses to be included in the BioMEL biobank were determined before launching the project. The aim is to acquire suitable tissue samples for every histopathological subdiagnosis to attain sufficient statistical power for translational research. Hence, 10–100 samples per histopathological subdiagnosis, dependent on how common the subdiagnosis is in Sweden and on formerly published research, were set as goals.2 Included diagnoses are presented in table 1.
Numbers and histopathological subdiagnoses for inclusion in BioMEL
Patients
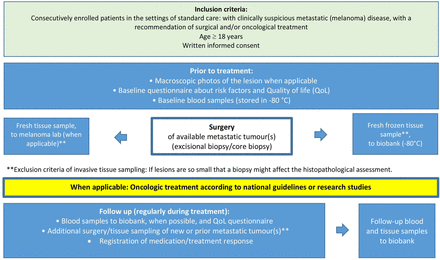
Patients ≥18 years planned for surgery due to an equivocal nevus, suspected CM or melanoma metastasis can be included for BioMEL research purposes in the mentioned hospitals in Region Skåne. All patients provide written informed consent. Blood samples and questionnaire data are collected and stored at inclusion (baseline) before diagnostic surgery. During surgical procedures (diagnostic excision/lymph node dissection/metastatic tumour excision/biopsy), tissue samples are collected. Tissue sampling is described below and presented in figures 1 and 2.
Overview of BioMEL study procedures for primary equivocal pigmented lesions. *Exclusion criteria of tape stripping: Ulcerated/bleeding/exudating lesion, topical treatment/sunscreen or moisturiser the last 24 hours, systemic treatment with steroids the last 30 days, known latex/tape allergy. **Exclusion criteria of invasive tissue sampling: The lesion area is so small that a punch biopsy of 1 mm diameter may affect the histopathological assessment.
Overview of BioMEL study procedures for metastatic melanoma lesions.
Questionnaire
At inclusion, patients answer a validated questionnaire,8 9 including information on medical history, prior UV exposure, family history of melanoma or other cancer types, skin type, diet, smoking habits, alcohol use and quality of life.
Blood samples, imaging and tissue samples collection
Blood samples (whole blood) are collected at inclusion before surgery (primary diagnostic excision or surgery for metastatic disease) (figures 1 and 2). In addition, further blood samples can be collected from patients with metastatic disease, especially during oncological treatment. Before the diagnostic excision of a primary lesion (nevus/melanoma), the included lesion is evaluated and registered using standard dermoscopic algorithms, mostly pattern analysis10 and the 7-point checklist algorithm.11 Primary lesions can be further documented by other imaging devices (eg, dermatoscopy devices, hyperspectral devices, confocal devices, optical coherence tomography (OCT) devices etc.) before and when applicable, also after excision. Tape sampling, or related non-invasive methods for harvesting biomarkers from the tumour surface before surgery, are other ethically approved possible diagnostics (figure 1).
Immediately after the local diagnostic excision of a primary pigmented skin lesion, full-thickness skin punch biopsies are taken by a trained dermatologist, guided by prior dermoscopic evaluation (figure 1). A 1 mm punch biopsy is taken in the most aggressive-looking area of the lesion. Still, not in the exact spot which, by the dermoscopic evaluation, is expected to be thickest.12 A 2 mm punch biopsy in unaffected skin adjacent to the tumour, is collected as a matched normal control. Tissue sampling is followed by prompt biobanking (snap freezing followed by −80°C storage). Biopsies can also be handled fresh and directly transported to the laboratory department in an appropriate medium. Tissue samples from suspected or confirmed melanoma metastases are collected through punch or core needle biopsies from surgically resected metastases. Tissue biopsies are preferably taken before, during and after oncological treatment or at an appropriate time for surgery of metastatic disease (figure 2). Biopsies can also be taken in case of disease progression or systemic treatment failure. Metastatic tissue samples are handled as described above for primary lesions but without prior dermoscopic evaluation. If additional surgery is planned, subsequent tissue samples can be collected for the BioMEL biobank.
Importantly, invasive tissue sampling for research purposes must never risk compromising histopathological diagnostics. Therefore, if the treating physician finds the suspected primary or metastatic CM size too small, patients will not donate tissue samples but can participate in all other parts of the BioMEL biobank (figure 2).
Clinical and histopathological information
Patient questionnaire data are stored in the BioMEL biobank database. Images, standardised for patients with a suspected primary CM include overviews/macroscopic views of the arms, thighs (back and front), trunk (back and front), the site of the lesion (close-up view), a close-up photo of the lesion and dermoscopic images of the lesion to be excised are stored. If other diagnostic imaging devices are used, the corresponding images from these diagnostic devices and photos of metastatic tumours are also stored. The histopathological information provided from the pathology report is registered in the same database, focusing on the mandatory information: length and width of the lesion (mm), type of lesion (nevi/melanoma/another diagnosis) and histopathological margins (mm). If the lesion is a nevus or a melanoma, further appropriate information is stored: detailed histopathological diagnosis, presence of dermal mitoses (yes/no), pathological T stage according to American Joint Committee on Cancer (AJCC) eighth edition staging system, Clark level, tumour thickness according to Breslow (mm) and if ulceration is present (yes/no).
Status of the BioMEL biobank
In June 2023, a total of 1205 registered lesions, questionnaires and corresponding tumour tissue samples, along with blood samples from the patients, were included in the BioMEL biobank: 705 tissue samples from primary lesions (660 patients) and 500 from metastatic lesions (458 patients).
Data and biobank quality assurance
The study database management is highly secured, in accordance with ethical approval. Two-factor authentication is used to access the biobank database; only approved BioMEL researchers can access the data.
The Swedish biobank law regulates the quality assurance of the collected materials in BioMEL. Written protocols about how biopsies should be taken ensure that the biopsies are safely collected and stored. Only registered research nurses or involved researchers handle the tissue biopsies in the freezers and the certified lab. The ISO-certified freezers (−80°C) are supervised by the biobank departments in the healthcare region (Region Skåne), all with alarms and backup systems.
Patient and public involvement
As clinical researchers and physicians, most individuals in the research group also have a professional relationship with the included research objects (patients). The patients can easily reach the principal investigators. Moreover, regular public events are held about our current research, where the public, and patients, are invited and can leave direct comments about the BioMEL biobank.
Ethics and dissemination
The BioMEL biobank project is approved by the national Swedish Ethical Review Authority (Dnr. 2013/101, 2013/339, 2020/00469, 2021/01432 and 2022-02 421-02) and is performed by the Declaration of Helsinki Ethical Principles for medical research, as well as by the Swedish Biobanks in Medical Care Act (SFS 2002:297). The datasets generated and analysed are not publicly available, as public sharing is not included in the current informed consent the patients have provided. Data can be available from the corresponding author on reasonable request. The BioMEL biobank has a steering group with senior researchers from the dermatology, surgery, oncology and melanoma genomics departments. The steering group handles all applications for possible research projects based on BioMEL data/tissue samples/blood samples. In addition, all separate applications must first be approved by the National Ethical Review Authority before sending an application to the BioMEL steering group. The motif and the planned use of the tissue/blood/data must be thoroughly described in the application to the steering group. The BioMEL steering group can decline projects if, for example, all tissue will be used, if projects do not fulfil the requirements in the primary ethical approval for the BioMEL biobank or if different applications involve the same research aims. Findings from future research generated from the BioMEL biobank will be distributed through peer-reviewed journals, media channels and conferences. The project is registered at ClinicalTrials.gov (NCT05446155).
Discussion
In this manuscript, we describe the BioMEL biobank: the implementation, setting and future perspectives of this biomedical edified data and biobank. The primary strength of the BioMEL biobank is its biological sample banks (blood and tissues), matched with the comprehensive and versatile clinical, molecular and image-based data, therefore, superior to other melanoma biobanks. Currently, several biobanks of CM exist or are under construction worldwide. According to our knowledge, there is no other research group that, since 2013, has collected both clinical and epidemiological data, medical and family history data, clinical and dermoscopic pictures, as well as blood samples and tissue samples—from very early stage CMs and equivocal nevi to more advanced and metastatic stages of CM. Well-known biobanks that collect information about CM through tissue samples include The Cancer Genome Atlas database13 and MelBase.14 However, these biobanks primarily include thick advanced and metastatic CM and thus do not have BioMEL’s advantage of also including the early stages of primary CM and its precursors.
The BioMEL biobank is a valuable research resource that will enable future molecular, histopathological, genetic and clinical marker studies. Not only are tissue samples of the suspected primary CM/metastasis collected, but another strength is that tissue samples of unaffected (adjacent) skin that act as matched controls are also collected. These matched tissue samples may allow investigation of the tumour’s microenvironment and possibly enable distinction of intraindividual and interindividual biological factors.
A significant challenge when taking invasive tissue samples in melanoma is the size and presentation of the tumour, especially when taking samples from primary CM. Sampling in tiny lesions (<5 mm in diameter) may disturb the histopathological assessment. However, excluding lesions with a diameter smaller than about 5 mm may lead to a selection bias. Another challenge is that a biopsy of 1 mm in diameter might not be representative of the final diagnosis/stage of the CM if it is taken in a more ‘benign’ part of the lesion. However, taking more voluminous tissue samples, or a sample where the melanoma has its highest Breslow thickness, could risk affecting diagnostics and subsequent treatment and follow-up of the patient. To avoid affecting staging and diagnostics, the physician performing the primary CM and equivocal nevi biopsy uses dermoscopy for guidance. With dermoscopy and palpation, melanoma thickness can be estimated and guide the physician on where to place the punch biopsy.12 To confirm the diagnosis of the area where the biopsy is taken, the assessing pathologists are asked to report if the tissue biopsy is taken from an area of the tumour that represents the final diagnosis. Since 2013, 3 out of >600 histopathological reports of the primary tumours examined have indicated that the biopsy might have impacted the histopathological evaluation. These cases were all thin melanomas where the pathologists noted that the biopsy was taken very close to the thickest part of the melanoma. In none of these cases, the diagnosis was influenced. Indeed, results from the BioMEL biobank will also indicate if the intralesional 1 mm biopsies have high enough tumour purity, and reporting whether a 1 mm biopsy will harvest enough tumour cells is essential to guide future research groups. Too little tumour cells could be one obstacle for sequencing, and our future experiences are important to share. A 2 mm punch biopsy device is used for normal skin tissue biopsies, a method which will also be evaluated in coming publications.
The BioMEL biobank is a prospective research project that will continue until the estimated total numbers of tumours are collected. Hence, some included patients could be reincluded and provide more tissue and blood samples if they develop multiple or metastatic melanomas. Likewise, patients with metastatic disease can later again provide samples after a recurrence of the disease and during oncological treatment. The prospective approach of the BioMEL biobank and the consecutive tissue and blood sampling strategies will enable us to study melanomas that are resistant to immunomodulatory therapy or tend to metastasise. Another strength of the BioMEL biobank is the population-based inclusion strategy. As CM constitutes a potentially fatal diagnosis, where knowledge gaps are apparent, and where enhanced objective and robust diagnostics could have a mortality-lowering impact, all stages of melanomas are essential. Thanks to the BioMEL biobank as a unique research resource for omics studies, potential biomarkers that can help us to differentiate equivocal nevi and early-stage CMs might be possible to find. The BioMEL biobank is a viable research platform for understanding factors related to CM overdiagnosis. In rare CM entities, for example, acral melanomas, the comprehensive information from BioMEL can complement existing registries/databases like the Swedish melanoma register.15 The BioMEL biobank also constitutes a base for evaluating responses to new therapies, like neoadjuvant therapy and for collaborations with other research groups.
Conclusion
The BioMEL biobank project constitutes a solid foundation for molecular studies regarding clinical and molecular characteristics and the evolution of nevi, primary CM and melanoma metastases. It might be used to understand novel and directed therapy outcomes and consequently improve patient survival. Imaging techniques and objective biomarkers in nevi and early melanoma will form a base for improved diagnostics of equivocal pigmented skin tumours in Swedish patients.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors Conceptualisation: KN, GJ and CI. Methodology: KN, GJ, VG, CI, KI, KH, AH, AC and BP. Investigation: all authors. Writing (original draft): TH, KN. Writing (review and critically editing): all authors. Supervision: KN, CI and GJ. Project Administration: KN. Funding Acquisition: KN, CI, GJ. All authors have read and agreed to the published version of the manuscript. Research nurses Marie Sjögren, Carina Eriksson and Catharina Nattoch Dag registered data and provided and cared for study patients.
Funding The BioMEL biobank and this work have been supported by the S.R. Gorthon Foundation, Hudfonden Foundation, Konung Gustaf V:s och Drottning Victorias Stiftelse, the Krapperup Foundation, the Liam M. Kinne Foundation, the Ingemar Persson foundation. Mats Paulsson Foundation, Stefan Paulsson Foundation, regional research grants (Regionala forskningsmedel) from Region Skåne, and the governmental funding for health care research (ALF) (grant number 2021-YF0069, grant numbers 2016-626231, 2020-0019 and 2022-0285).
Disclaimer The funders have no role in any aspects of the methodology, writing the manuscript or decision to submit the protocol for publication.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.