Article Text
Abstract
Introduction Previous randomised controlled trials (RCTs) have indicated a protective role of pregnancy supplementation with fish oil and high-dose vitamin D, respectively, on offspring asthma, infections and several other disorders in early childhood. However, current evidence is not considered sufficient for recommending these supplements in pregnancy. In two RCTs, we aim to investigate whether these protective effects can be confirmed in larger trials with the goal of changing clinical practice and improving child health.
Methods and analysis Randomisation of 4000 pregnant women to either (1) (n=2000) the fish oil trial of 2.4 g/day (55% eicosapentaenoic acid (EPA) and 37% docosahexaenoic acid (DHA)) in triacylglycerol form versus placebo or (2) (n=2000) the vitamin D trial of high-dose (3200 IU/day) vitamin D versus placebo on top of the recommended 400 IU/day. Supplementation begins in gestational week 24 (22–26) until 1 week after delivery. Allocation to the trials will be determined based on the preinterventional maternal blood levels of EPA+DHA with a dried blood screening test. Women with low levels (below 4.7% of total fatty acids) will be assigned to the fish oil RCT, and women with high levels will be assigned to the vitamin D RCT. Maternal blood will be used for genetic, metabolomic and proteomic profiling. A 3-year follow-up of the children with longitudinal registration of parent-reported symptoms, diagnoses, medication use and hospitalisations will be performed. The primary outcome is persistent wheeze or asthma until age 3 years, with predefined analyses of effect modification by maternal genotypes. Secondary outcomes are lower respiratory tract infections, gastrointestinal infections, croup, troublesome lung symptoms, eczema, allergy, bone fractures, developmental milestones, mental health, cognition and growth until age 3 years. A follow-up on both primary and secondary outcomes is planned after unblinding, from age 3–6 years.
Ethics and dissemination The RCTs are approved by the Danish local ethics committee (H-23055833). The studies are registered at ClinicalTrials.gov (NCT06560255 and NCT06570889). Study results will be communicated to the medical community, including publications in peer-reviewed journals. All results will be published and available on www.copsac.com.
Trial registration number NCT06560255 and NCT06570889.
- Child
- Asthma
- NUTRITION & DIETETICS
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Two large randomised controlled trials (RCTs) consisting of two oral micronutrient interventions of fish oil and high-dose vitamin D in pregnant women investigate whether prenatal supplementations have beneficial effects on the risk of persistent wheeze or asthma in the offspring.
The RCTs target a personalised prevention strategy by analysing the added value of specifically targeting pregnant women with FADS genetic risk variants for fish oil intervention and 17q21, VDR and VDBP genetic risk variants for vitamin D supplementation.
The RCTs investigate the underlying mechanisms in additional exploratory studies using proteomic and metabolomic profiling of the pregnant mothers in relation to the potential intervention effects.
The RCTs are highly dependent on adherence to the supplementations and the parent’s registration of symptoms of the children.
Introduction
Asthma is a chronic inflammatory disorder characterised by hyperresponsiveness of the airways. The prevalence has more than doubled in Westernised societies over the recent decades,1 and asthma and wheeze are currently the main reasons for chronic medication use and hospitalisation of young children having a huge impact on children and their families.2 Interestingly, a concomitant rise in the use of vegetable oils has been observed, which has resulted in an increase in the intake of n-6 polyunsaturated fatty acids and a decrease in the intake of n-3 polyunsaturated fatty acids (n-3 LCPUFAs)—eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).3 Further, a parallel rise in vitamin D deficiency has been observed.4 n-3 LCPUFA and vitamin D have been associated with a range of protective mechanisms in relation to early childhood asthma occurring before school age,5–7 and observational studies8–11 have suggested that low pregnancy intake of n-3 LCPUFAs and vitamin D are associated with a higher overall risk of wheezing in early childhood.
In a previous randomised controlled trial (RCT) in the Copenhagen Prospective Studies on Asthma in Childhood 2010 (COPSAC2010) of 700 mother–child pairs, we found evidence that fish oil supplementation (2.4 g (55% EPA and 37% DHA) of n-3 LCPUFA vs matching olive oil capsules) reduced the risk of asthma in the offspring by age 5 years.3 The effect was most pronounced in mothers with low EPA+DHA levels, that is, below the median of 4.7% of total fatty acids in erythrocyte equivalents. We further found a protective effect of fish oil on the child’s risk of lower respiratory tract infections,3 gastroenteritis,12 croup13 and neurodevelopment in the boys.14 Unexpectedly, we observed that fish oil supplementation was associated with increased offspring body mass index (BMI) from birth to age 10 years.15 Others have demonstrated supplementation with n-3 LCPUFA to be both promising and safe in reducing preterm and early preterm birth in women with low blood levels of n-3 LCPUFA.16–18
In addition, we previously demonstrated a protective effect from high-dose vitamin D (2800 IU/day and 4400 IU/day vs 400 IU/day) on episodes of troublesome lung symptoms at age 0–3 years19 and, in a meta-analysis combining the data from COPSAC2010 with a similar vitamin D RCT (VDAART),20 a protective effect on persistent wheeze until age 3 years.21 Interestingly, the effect was largest in mothers having high entry levels of 25 hydroxyvitamin D (25(OH)D).21 Further, high-dose vitamin D seemed to affect the child’s airway microbial colonisation in early life22 and the airway immune profile was upregulated in these children.19 We and others also observed vitamin D supplementation during pregnancy to have a protective effect on lower respiratory tract infections and croup.13 20 Lastly, in the COPSAC2010 RCT, we observed improved bone mineralisation until age 6 years and a reduced risk of fractures in children born to mothers receiving high-dose vitamin D intervention.23
The above RCTs are so far the only well-powered large-scale fish oil (COPSAC2010) and vitamin D (COPSAC2010 and VDAART) supplementation trials in pregnancy showing protective effects on early childhood asthma as the primary endpoint, and further evidence is therefore needed to provide the basis for recommending these supplements to pregnant women.
In the current RCTs, our aim is to investigate whether the beneficial effects can be confirmed in two larger RCTs with the goal of changing clinical practice. Our previous results indicated that the two interventions influence each other, so that the combined effect of two interventions was not superior to giving one intervention alone.13 We will therefore allocate mothers to only one intervention, either the fish oil or the vitamin D trial.
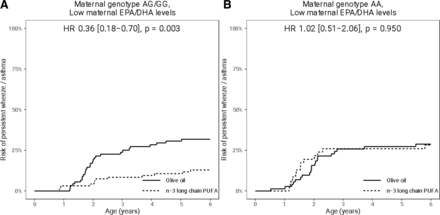
Mothers with low levels of EPA+DHA will be allocated to the fish oil trial. We have chosen the median level as cut-off, and stratified analyses based on this show the highest effect from fish oil supplementation on persistent wheeze or asthma in a woman with low EPA+DHA level in line with previous reports3 (figure 1). In our previous trial, an even larger effect was seen among mothers with low levels who also carried specific fatty acid desaturase (FADS) gene variants (figure 2), and this potential tool for further precision prevention will be analysed.
The effect of fish oil supplementation on the risk of persistent wheeze/asthma during the first six years of life stratified by maternal blood levels below (A) or above (B) the population median of eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) blood levels at pregnancy week 24 (EPA+DHA=4.7% of total fatty acid levels). Based on data from Bisgaard et al.3
Effect of fish oil supplementation on the risk of persistent wheeze/asthma during the first six years of life among women with low eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) blood levels and further stratified on (A) at least one fatty acid desaturase (FADS) (rs1535) risk allele and (B) no FADS risk alleles. Based on data from Bisgaard et al.3
Pregnant women with high entry levels of EPA+DHA will be allocated to the vitamin D trial. As a group, these women are expected to have the highest entry levels of 25(OH)D and are therefore expected to have a high effect from vitamin D intervention.21
Methods and analysis
The studies are two double-blinded RCTs (NCT06560255 and NCT06570889) with two interventions of either n-3 LCPUFA or high-dose vitamin D. The aim is to study the (1) clinical effects of supplementations on early childhood asthma/persistent wheeze (primary outcome) and secondary outcomes, and (2) analyse the potential added value of personalised approaches for disease prevention specifically targeting women with FADS genetic risk variants for fish oil intervention and vitamin D receptor (VDR), vitamin D-binding protein (VDBP) and 17q21 genetic risk variants for vitamin D supplementation, and other common gene variants in more exploratory approaches using polygenic risk scores and pathway-based analyses.
Participants, interventions and outcomes
Study population and eligibility
We will initiate the recruitment of Danish pregnant women into the study from Q4, 2024. Based on the mother’s EPA+DHA blood levels observed in the previous COPSAC2010 study, we plan to recruit approximately 4000 pregnant women, which will include approximately 2000 women with low blood levels of EPA+DHA in the fish oil trial and 2000 mothers with high blood levels of EPA+DHA in the vitamin D trial. In both trials, the women will be randomised in a 1:1 setting (figure 3).
Overview of the study procedure.
The inclusion criteria of the fish oil trial:
Pregnant Danish women before gestational week 26 with blood levels of EPA+DHA below 4.7% of total fatty acids
No planned use of fish oil supplementations
No endocrine, heart or kidney disorders
The inclusion criteria of the vitamin D trial:
Pregnant Danish women before gestational week 26 with blood levels of EPA+DHA above or equal to 4.7% of total fatty acids
No current vitamin D intake above the recommended 400 IU/day
No endocrine, heart or kidney disorders
Recruitment
Pregnant women will be recruited from the lists of general practitioners’ reimbursements of women’s first pregnancy consultation. The reimbursement list is accessed by the COPSAC personnel, allowing us to identify pregnant women early in their pregnancy and contact them directly. This approach was used successfully in the previous COPSAC2010 study and will allow us to recruit pregnant women before the third trimester and initiate the intervention around week 24 (weeks 22–26) of pregnancy. The pregnant women will be invited to contact the COPSAC research unit for further information. If the women wish to hear more about the study, they will be directed to a landing page via a link, where they can register to receive the participant information and book a time for the inclusion interview.
Interventions
The two interventions are dependent on the baseline EPA+DHA levels and are as follows:
Fish oil of 2.4 g/day (55% EPA and 37% DHA) in triacylglycerol form or placebo (rape seed oil) in 2000 women with low blood levels of EPA+DHA.
Or
High-dose (3200 IU/day) vitamin D or placebo on top of the recommended 400 IU/day in 2000 women with high levels of EPA+DHA
Interventions will begin at enrolment before week 26 and continue until 1 week after delivery. Both investigators and participants will remain unaware of group assignments until the youngest child in the trial reaches 3 years of age.
A follow-up at age 3–6 years is planned for both primary and secondary outcomes.
Information on supplementations
Fish oil
Active treatment: 2.4 g per day of fish oil (55% EPA and 37% DHA) in triacylglycerol (GoldenOmega, PharmaNord, DK).
Placebo: rape seed oil (PharmaNord, DK)
Both fish oil and placebo are administered in four 1 g capsules.
Vitamin D
Active treatment: 3200 IU/day (80 μg) vitamin D3 (cholecalciferol) (D-Pearls, PharmaNord A/S) + additional 400 IU/day (10 μg).
Placebo: Placebo Pearls from PharmaNord A/S with no active ingredients + additional 400 IU/day (10 μg).
Outcomes
Primary outcome
Persistent wheeze or asthma age 0–3 years defined as either
Parental report of a minimum of two wheeze episodes with at least one after the child’s second birthday and redemption of at least two prescriptions of asthma controller medication with at least one being after the child’s second birthday.
Or
A minimum of two emergency department (ED) visits or hospitalisations due to asthmatic symptoms and at least one of these being after the child’s second birthday.
Or
Parental report of physician-diagnosed asthma age 0–3 years.
Outcome analyses: risk (survival analysis) of persistent wheeze or asthma age 0–3 years.
Secondary outcomes
Atopic/non-atopic wheeze or asthma age 0–3 years based on parental reports of physician-diagnosed asthma in combination with eczema and/or allergic rhinitis.
Outcome analyses: asthma/persistent wheeze with/without eczema and/or allergic rhinitis (yes/no) by age 3 years. Each subtype is compared with children without asthma.
Troublesome lung symptoms age 0–3 years defined as cough, wheeze or dyspnoea severely affecting the well-being of the child for at least three consecutive days based on parental reports.
Outcome analyses: total no. of episodes with symptoms age 0–3 years.
Wheeze episodes age 0–3 years based on parental reports.
Outcome analyses: total no. of episodes with wheeze age 0–3 years.
Inhaled bronchodilator use age 0–3 years from parental reports.
Outcome analyses: total no. of days with bronchodilator use age 0–3 years.
Asthma controller medication use age 0–3 years (inhaled corticosteroids and leukotriene modifiers) based on parental reports.
Outcome analyses: total no. of days with the use of asthma controller medication age 0–3 years.
Asthma controller medication 0–3 years from redeemed medication prescriptions (inhaled corticosteroids and leukotriene modifiers) based on medical record checks:
Outcome analyses: total no. of prescriptions age 0–3 years.
Inhaled bronchodilators from redeemed inhaled bronchodilators based on medical record checks:
Outcome analyses: total no. of prescriptions age 0–3 years.
Asthma hospitalisations or ED visits age 0–3 years based on medical record checks.
Outcome analyses: total no. of episodes age 0–3 years.
Eczema age 0–3 years based on parental reports of physician-diagnosed eczema and prescribed topical anti-inflammatory medication on medical record checks.
Outcome analyses: risk (survival analysis) of any eczema age 0–3 years and eczema (yes/no) age 0–3 years.
Allergic rhinitis age 0–3 years based on parental reports of physician-diagnosed allergic rhinitis.
Outcome analyses: allergic rhinitis (yes/no) by age 3 years.
Food allergy age 0–3 years based on parental reports of physician-diagnosed food allergy.
Outcome analyses: any food allergy (yes/no) by age 3 years.
Lower respiratory tract infections age 0–3 years based on parental reports of physician-diagnosed bronchiolitis or pneumonia.
Outcome analyses: risk (survival analysis) of first lower respiratory tract infection and total no. of lower respiratory tract infections age 0–3 years.
Gastrointestinal infections age 0–3 years based on parental reports.
Outcome analyses: risk (survival analysis) of any gastroenteritis episode and total no. of gastroenteritis episodes age 0–3 years.
Croup age 0–3 years based on parental reports of physician-diagnosed croup.
Outcome analyses: risk (survival analysis) of any croup episode and total no. of croup episodes age 0–3 years.
Fever episodes age 0–3 years based on parental reports.
Outcome analyses: total no. of episodes with symptoms age 0–3 years.
Absences from daycare due to illnesses age 0–3 years based on parental reports.
Outcome analyses: total no. of absence days age 0–3 years.
Fractures assessed by medical record checks, including all radiologically verified fractures of larger long bones (ie, clavicle, radius, ulna, tibia, fibula, femur and humerus).
Outcome analyses: total no. of fractures age 0–3 years.
Developmental milestones age 0–3 years monitored every 6 months by the parents using a registration form based on the Denver Development Index and WHO milestones registration.
Outcome analyses: combined assessment of age at achievement across milestones by principal component analysis.
Strength and difficulties at age 3 years from the Strengths and Difficulties Questionnaire (SDQ), which is a brief behavioural screening questionnaire.
Outcome analyses: SDQ scores at age 3 years.
Attention deficit hyperactivity disorder (ADHD) symptoms at age 3 years from the ADHD rating scale IV preschool, a dimensional questionnaire for ADHD symptoms in preschool children.
Outcome analyses: ADHD symptom scores at age 3 years.
Social behaviour assessment at age 3 years from the Social Responsiveness Scale, second edition; a questionnaire for screening for autistic traits:
Outcome analyses: total problem scores at age 3 years.
Psychopathology screening at age 3 years from the Child Behaviour Check List 1.5–5 years; a questionnaire for general screening of preschool psychopathology.
Outcome analyses: total syndrome scales at age 3 years.
Cognitive assessment by age 3 years from Behaviour Rating Inventory of Executive Function in preschool children; a measurement of executive functions.
Outcome analyses: index values at age 3 years.
BMI and waist circumference age 0–3 years from parental reports every 6 months.
Outcome analyses: development of BMI and waist circumference age 0–3 years and current BMI and waist circumference at age 3.
Outcomes in the follow-up to age 6 years
Persistent wheeze or asthma age 0–6 years, defined similarly to the primary outcome.
Outcome analyses: risk (survival analysis) of persistent wheeze or asthma at age 0–6 years.
Current asthma at age 6 years, defined similarly to the primary outcome and with symptoms and/or asthma medication use in the last 12 months at age 6 years.
Outcome analyses: current wheeze or asthma (yes/no) at age 6 years.
Asthma or wheeze yearly prevalence age 0–6 years with any wheeze/asthma defined as in the primary outcome and current disease defined from parental reports of wheeze and/or asthma medication use.
Outcome analyses: yearly prevalence of wheeze or asthma medication use (yes/no) age 0–6 years.
Atopic/non-atopic asthma at age 6 years based on parental reports of physician-diagnosed asthma in combination with eczema and/or allergic rhinitis.
Outcome analyses: current asthma with/without eczema and/or allergic rhinitis (yes/no) by age 6 years. Each subtype is compared with children without asthma.
Asthma controller medication age 0–6 years from redeemed medication prescriptions (inhaled corticosteroids and leukotriene modifiers) based on medical record checks:
Outcome analyses: total no. of prescriptions age 0–6 years.
Inhaled bronchodilators age 0–6 years from redeemed inhaled bronchodilators based on medical record checks:
Outcome analyses: total no. of prescriptions age 0–6 years.
Asthma hospitalisations or ED visits age 0–6 years based on medical record checks.
Outcome analyses: total no. of episodes age 0–6 years.
Eczema age 0–6 years based on parental reports of physician-diagnosed eczema.
Outcome analyses: risk (survival analysis) of any eczema age 0–6 years and current eczema (yes/no) by age 6 years.
Allergic rhinitis age 0–6 years based on parental reports of physician-diagnosed allergic rhinitis and on medical record checks.
Outcome analyses: allergic rhinitis (yes/no) by age 6 years.
Food allergy age 0–6 years based on parental reports of physician-diagnosed food allergy.
Outcome analyses: any food allergy (yes/no) by age 6 years.
Lower respiratory tract infections age 0–6 years based on parental reports of physician-diagnosed bronchiolitis or pneumonia.
Outcome analyses: risk (survival analysis) of first lower respiratory tract infection and total no. of lower respiratory tract infections age 0–6 years.
Gastrointestinal infections age 0–6 years based on parental reports.
Outcome analyses: risk (survival analysis) of any gastroenteritis episode and total no. of gastroenteritis episodes age 0–6 years.
Croup 0–6 years based on parental reports of physician-diagnosed croup.
Outcome analyses: risk (survival analysis) of any croup episode and total no. of croup episodes age 0–6 years.
Fractures 0–6 years assessed by medical record checks, including all radiologically verified fractures of larger long bones (ie, clavicle, radius, ulna, tibia, fibula, femur and humerus).
Outcome analyses: total no. of fractures age 0–6 years.
Strength and difficulties at age 6 years from the SDQ, which is a brief behavioural screening questionnaire.
Outcome analyses: SDQ scores at age 6 years.
ADHD symptoms at age 6 years from the ADHD rating scale IV preschool, a dimensional questionnaire for ADHD symptoms in preschool children.
Outcome analyses: ADHD symptom scores at age 6 years.
Social behaviour assessment at age 6 years from the Social Responsiveness Scale, second edition; a questionnaire for screening for autistic traits:
Outcome analyses: total problem scores at age 6 years.
Psychopathology screening at age 6 years from the Child Behaviour Check List; a questionnaire for general screening of preschool psychopathology.
Outcome analyses: total syndrome scales at age 6 years.
Cognitive assessment by age 6 years from Behaviour Rating Inventory of Executive Function in preschool children; a measurement of executive functions.
Outcome analyses: Index values at age 6 years.
BMI and waist circumference from birth to age 6 years from parental reports every 6 months.
Outcome analyses: development of BMI and waist circumference age 0–6 years and current BMI and waist circumference at age 6.
Assessment of outcomes and baseline characteristics
The women/families will be instructed on how to answer questionnaires in Research Electronic Data Capture (REDCap), a secure web application for building and managing online surveys and databases. This will be used for registering baseline characteristics and outcomes throughout the study.
At the enrolment, the women will answer a questionnaire about dietary habits, parental level of education and history of asthma, allergies and eczema for themselves and siblings to the unborn child. At birth, the parents are interviewed on pregnancy and birth-related questions such as postnatal environment of the child, thus providing an overview of the most important constituents of the exposome of importance for asthma risk such as mode of delivery, household pets, breastfeeding and tobacco smoke exposure.
The primary outcome, persistent wheeze or asthma, is defined to resemble the diagnostic algorithm used in the previous COPSAC2010 study based on recurrent asthma-like symptoms with a certain duration and demonstrated effect of inhaled corticosteroids.3 In the current study, where the diagnosis and treatment of asthma are done outside the research clinic, persistent wheeze or asthma is defined by at least one of the following: (1) parental report of a minimum of two wheeze episodes with at least one after the child’s second birthday and redemption of at least two prescriptions of asthma controller medication (defined as steroid inhalers or leukotriene modifiers) with at least one being after the child’s second birthday. At least 3 months are required between the first and last prescription to emphasise disease with a certain duration and apparent treatment effect; or (2) a minimum of two ED visits or hospitalisations due to asthmatic symptoms documented from medical records and at least one of these being after the child’s second birthday; or (3) parental report of physician-diagnosed asthma age 0–3 years. The first date of fulfilling any of these three criteria are used in the survival analysis.
A current diagnosis is defined as fulfilling the diagnosis and still having symptoms and/or treatment in the last year.
In the first three years of life, the families will register asthma symptoms, infections, fever and absence from daycare every fortnight in REDCap. From age 3–6 years, information on lower respiratory tract infections, croup and gastroenteritis will be registered in REDCap every 6 months.
The families are asked to answer questions about height, weight, waist circumference, physician-diagnosed asthma, allergic, rhinitis, food allergy and eczema every 6 months.
The child’s medical prescriptions, hospital diagnoses and vaccine records are collected through the national health registers from birth to age 6 years to capture information related to the outcomes. These will also be used to capture information on maternal diseases and medical treatment during pregnancy and perinatally in order to document safety of the intervention.
The families are asked to fulfil questions on mental health at age 3 and 6 years in terms of strength and difficulties from the SDQ, a brief behavioural screening questionnaire; ADHD symptoms from the ADHD rating scale IV preschool, a dimensional questionnaire for ADHD symptoms in preschool children; social behaviour from the Social Responsiveness Scale, second edition, a questionnaire for screening for autistic traits; psychopathology from the Child Behaviour Check List 1.5–5 years, a questionnaire for general screening of preschool psychopathology; and cognitive assessment from Behaviour Rating Inventory of Executive Function in preschool children, a measurement of executive functions.
Developmental milestones are monitored every 6 months until age 3 years by the parents using a registration form based on the Denver Development Index and WHO milestones registration.
Research assistants and PhD students will monitor the registered data and send out reminders and/or contact the families by telephone to ensure a high level of registration.
Blood level assessment for assignment to treatment arm
An EPA+DHA blood screening test for self-testing will be sent to participants and thereafter analysed within a week at Vitas, Norway. The women with low EPA+DHA levels (below 4.7% of total fatty acids) will be assigned to the fish oil RCT, and the women with high levels of EPA+DHA will be assigned to the vitamin D RCT. The supplements will be sent to the participants from the research facility by courier post.
After pregnancy/intervention, the mother will perform a similar self-testing blood test for the assessment of blood EPA+DHA/vitamin D levels.
Data collection, management and analysis
Biological samples and storage
Measurement of fatty acid and 25(OH)D blood levels in pregnant women
The profiling of fatty acids and 25(OH)D levels in pregnant women’s blood will be performed within 1 week after recruitment and 1 week after giving birth. The analyses will be performed by Vitas AS (Norway) using a validated home testing setup. Service agreement, data processing agreement and material transfer agreement will be made with Vitas to comply with the Danish data protection laws.
Capillary blood (less than 1 mL) is collected from the fingertip using a dry blood spot kit. A panel of 20 fatty acids will be measured, including saturated fatty acids, monounsaturated fatty acids, n-3 LCPUFAs (including EPA and DHA) and n-6 LCPUFAs. The filter paper will be stored for later analyses of 25(OH)D levels at the Danish Statens Serum Institute with setup for these analyses on dried blood spots.
Genetic, metabolomic and proteomic profiling of the mothers
The remaining blood from the dried blood spots after fatty acid and 25(OH)D assessments will be used for genetic, metabolomic and proteomic profiling.
Genome-wide genotyping will be conducted with the primary aim to analyse genetic variants in genes of importance for the n-3 LCPUFA, including single-nucleotide polymorphism (SNP) rs1535 and vitamin D metabolism including SNP rs12936231, but also for more exploratory analyses of gene pathways and polygenic risk scores potentially modifying the treatment response and thereby allowing a more refined precision prevention approach. Genome-wide genotyping will be performed using a modified version of the Illumina GSA array, assuring that rare variants and ‘actionable’ gene variants on the American College of Medical Genetics list are not generated. This procedure avoids incidental findings of gene variants of clinical significance at the individual level.
Metabolomic profiling will also be conducted to assess the dietary profile of the mothers and the underlying mechanisms of the interventions. This will be done using an in-house method developed at the Statens Serum Institut specifically for use on dried blood spots.24
Proteomics will be performed to understand the inflammatory process targeted by the pregnancy interventions. This will be done using inflammatory panels from the company O-link (Sweden). The choice of panels will depend on prices at the time of analyses, but the current planned panel is the Inflammation 96 panel including 96 inflammatory proteins.
Both the genotyping, proteomic and metabolomic profiling will be done using the same blood sample (stored dried blood spot) also used for the EPA, DHA and 25(OH)D levels. The blood samples will be stored in a biobank at Gentofte Hospital (Denmark) and later at the Danish National Biobank at Statens Serum Institute until they are used for analyses. The samples will only be used for this project and the remaining material will be destroyed when the project period ends. If the participants want to retract their participation in the study, biological samples will be destroyed.
Statistical power calculation for each intervention arm
The study sample size is based on power calculations for the primary outcome (early childhood asthma/persistent wheeze). Based on the findings from the population-based COPSAC2010 cohort,3 we assume that 20% of the children will develop this outcome in the first three years of life. With a conservative estimated risk reduction of 30%, a significance level of 0.05 and a power of 90%, the required sample size will be 1643 children. With an estimated 5% dropout among recruited pregnant women and a further 10% dropout of children before age 3 years, the number of recruited pregnant women should be 1933 in each of the RCTs.
Statistical methods
We will analyse baseline characteristics to describe the cohorts and make sure that the randomisation has been successfully using Student’s t-test and χ2 tests. The effect of fish oil and the high-dose vitamin D interventions on age at onset endpoints, that is, persistent wheeze, lower respiratory tract infections, gastrointestinal infections, croup and eczema, will be assessed by Kaplan-Meier curves and quantified by Cox proportional hazards regression (p values corresponding to Wald tests). The main analysis will be performed as an intention-to-treat analysis. The children will be retained in the analysis from birth until the age of diagnosis, dropout or age at their last clinic visit at completion of the RCT, whichever came first. The effect on dichotomised endpoints, that is, persistent wheeze/asthma, eczema and allergic rhinitis, will be analysed with logistic regression. The effect on count endpoints, that is, lower respiratory tract infections, gastrointestinal infections, croup, troublesome lung symptoms and fractures, will be analysed using Quasi-Poisson regression. The effects on continuous endpoints, that is, milestones, ASQ-3, SDQ, BMI and waist circumference, will be analysed using linear model and BMI, and waist circumference will also be analysed using repeated measurements mixed linear models.
Effect modification from specific gene variants will be analysed based on previous findings, including rs1535 for the fish oil intervention3 and rs12936231 for vitamin D.25
The effects of fish oil and the high-dose vitamin D interventions will be evaluated for clinical benefit as the number needed to treat. Results with a p value<0.05 will be considered significant. Missing data will be treated as missing observations. Data processing will be conducted using the statistical software R.
Administration and safety
Fish oil supplementation
The previous Danish COPSAC RCT including 736 pregnant women demonstrated that fish oil supplementation during the third trimester was feasible as 71% had taken >80% of the planned dosages.7 The content of EPA+DHA fatty acids in the blood is primarily determined by dietary consumption of oily fish. Current national and international recommendations on fish intake during pregnancy typically encourage women to consume two servings of oily fish per week, while the supplement used in the COPSAC RCT amounted to a total of 2.4 g fish oil per day equalling four servings of oily fish per day. Evidence from other large-scale studies suggests that prenatal supplementation with fish oil reduces the risk of preterm birth in women with low baseline EPA+DHA levels, while supplementation might increase the risk of preterm birth in women with high baseline levels,16 26 supporting the approach of only providing supplements to women with low levels.
Concerns have been raised that the perturbation of the eicosanoid cascade may cause unfavourable changes in the balance of some mediators with hemostatic effects possibly causing excessive bleeding during delivery. Our previous fish oil trial of 2.4g n-3 LCPUFA supplementation in pregnancy revealed no adverse effects in the mothers or children.7 The WHO states: “Fish oil supplements do not appear, however, to cause any serious side effects such as bleeding complications or discomfort that would influence compliance issues other than the rather minor complaint of unpleasant taste”.27 28
One potential putative side effect is an increased risk of induced labour due to the prolonged gestation caused by n-3 LCPUFA supplementation. However, this was not evident in the previous COPSAC trial. In the proposed project, we also investigate the safety profile of n-3 LCPUFA supplementation through hospital-registered birth complications as well as by having the women register any observed side effects from the fish oil supplementation.
In our previous COPSAC2010 trial, we observed increased BMI by age 6 years in children of mothers who received fish oil.14 This was an unexpected finding not found in previous fish oil trials and could be a chance finding. This emphasises the need for a larger randomised trial, also to investigate potential negative health effects related to fish oil supplementation during pregnancy.
Vitamin D supplementation
In a review of vitamin D intervention studies, it was concluded that doses ≤10 000 IU vitamin D/day (250 µg/day) for up to 5 months did not elevate 25(OH)D levels above 225 nmol/L.29 Including only pregnant women and their children, a recent meta-analysis was conducted of vitamin D intervention trials, which showed no increased risk of adverse events (AEs) in children (n=3780), including studies with supplementation doses of up to 5000 IU/day during pregnancy from week 20 until birth.30 In this specific study, they found that the supplementation of 5000 IU/day throughout pregnancy was well tolerated and highly effective at preventing neonatal vitamin D deficiency.31
In both the previous COPSAC201019 and VDAART20 trials, the supplementations (2800 IU/day from week 24 until birth and 4400 IU/day from week 14 until birth) of vitamin D during pregnancy were not associated with any of the safety outcomes measured in both mothers and children. In addition, no events of hypercalcaemia occurred in the mothers from vitamin D supplementations of 4400 IU/day.20 The daily recommended intake of vitamin D described in the Danish national guideline is currently defined as 400 IU/day (10 µg) during pregnancy to reach a suggested threshold of 50 nmol/L and is in line with recommendations from the Institute of Medicine to prevent bone-related diseases. However, emerging evidence suggests that this supplementation regime is too low to reach the thresholds of any beneficial effects on asthma symptoms in children. Interestingly, the recommendations of a threshold of 75 nmol/L from the Endocrine Society are based on evidence showing up to a 65% increase in calcium absorption when going from 50 to 75 nmol/L, supporting the potential need for an updated vitamin D intake guideline why they conclude that at least 1500–2000 IU/day during pregnancy is needed to maintain a blood level above 75 nmol/L.32 As the fetus is not able to synthesise 25(OH)D, it highly relies on placental transfer, and a previous RCT study of supplementation doses of up to 4000 IU/day through pregnancy showed that the highest 25(OH)D concentration in neonates after birth from this supplementation group was 130 nmol/L, which is far from the level of 200 nmol/L that is now considered to hold a potential risk of adverse health outcomes in infants by the European Food Safety Authority (EFSA).33
The most well-known potential side effect of toxic levels of vitamin D and selected by the EFSA as the indicator of vitamin D toxicity is hypercalcaemia. From the VDAART trial supplementing with 4400 IU/day from week 14 until birth, no cases of hypercalcaemia were reported.20 In the RCT study by Yap et al, where women were randomised to either 5000 IU/day or 400 IU/day of vitamin D through pregnancy, no maternal hypervitaminosis D (>250 nmol/L) was observed, and there were no statistical differences between the two groups in cord-blood safety measurements.31 Known potential side effects of vitamin D intoxication other than hypercalcaemia are nausea, vomiting, poor appetite and weight loss, weakness, kidney stones and disorientation. However, these have not been related to dose ranges below the EFSA upper tolerable level and only in studies with intake of more than 10 000 IU/day and where levels of 25(OH)D of above 250–700 nmol/L were registered.34–36
We will use 3200 IU/day on top of the recommended 400 IU/day, totalling a maximum of 3600 IU/day, which is below the EFSA-defined upper tolerable level for pregnant women (4000 IU/day). We consider this dose to be safe. We only include women who are not taking any high-dose vitamin D supplements before enrolment to ensure no risk of toxic levels due to high baseline levels and with no endocrine diseases that are considered to be of any risks. Other sources of vitamin D from sunlight exposure and food intake are not considered holding potential risks of intoxication, and by excluding women with a high current intake of supplementations, we consider that there are no potential health risks by these administrations. Our dose and inclusion criteria in this vitamin D trial are also in line with a previously approved Danish RCT study GRAVITD (NCT04291313), which is currently recruiting participants assessing the effect of 3600 versus 400 IU/day of vitamin D from the first trimester on adverse pregnancy outcomes.
Safety procedures
Registration of AEs, which are any unexpected and unfavourable sign(s) within the pregnant woman or child that could potentially be caused by the supplements, will be registered. Similarly, any serious AEs that are any AEs causing death or hospitalisations will be registered.
Ethics and dissemination
Oral fish oil and vitamin D supplementation in pregnant women have been shown to be safe for both the mother and the child in previous studies.16 30 Hence, we consider the administration of 2.4 g of n-3 LCPUFA or 3200 IU of vitamin D daily in late pregnancy to be associated with minimal or no health risks. In general, pregnant women are low in 25(OH)D levels due to a high need for vitamin D during pregnancy and the fetus being dependent on the mother’s intake. The current national guidelines already recommend a daily dose of vitamin D throughout the pregnancy, but the currently recommended dose has been shown to be insufficient for ensuring sufficiently high doses for a large number of pregnant women and would not ensure the levels indicated to improve health in the child in previous studies. A higher BMI in offspring of mothers receiving fish oil supplementation was found in COPSAC2010,15 but was unexpected from experimental studies and not found in another RCT using similar doses,37 and could therefore be a spurious finding. Thus, we consider this trial not to be associated with ethical concerns, but instead expect the interventions to be beneficial for both the mother and the child. The blood analyses, including genetics, metabolomics and proteomics, do not include data with direct health implications for the individual and are therefore not considered an ethical concern. The RCTs are approved by the Danish local ethics committee (H-23055833).
The two studies are registered at ClinicalTrials.gov (NCT06560255 and NCT06570889). We will ensure that the research results will benefit the future generation of children by having the information communicated to the medical community via national/international conferences and publications in peer-reviewed journals, to lay people via social and public media and to families of preschoolers with asthma by involving patient organisations and networks. All results will be published. Further, the results will be available on our website www.copsac.com.
Perspectives
If the previously reported beneficial health effects from fish oil and high dose vitamin D in pregnant women are confirmed, these trials could provide the basis for changing current nutritional guidelines for pregnant women and improving child health.
The study design is aiming at developing a personalised prevention strategy. Since our previous results have indicated that the two interventions could influence each other with a potential ceiling effect, we employ a stratified intervention approach. Mothers with low levels of n-3 LCPUFA (EPA+DHA) will be allocated to the randomised fish oil study, where we also expect the highest intervention effect on wheeze and asthma based on the findings from our previous trial.3 Mothers with high EPA+DHA levels will be allocated to the vitamin D study. As a group, these women will have higher levels of 25(OH)D and are therefore expected to benefit from vitamin D supplementation, as we previously found the largest effect with high vitamin D levels.21 If both trials are successful, this approach could be implemented in the clinical setting, ensuring a preventive approach for all pregnant women.
Based on our previous findings, we further analyse the potential added value of specifically targeting pregnant women with FADS genetic risk variants for fish oil intervention and VDR, VDBP and 17q21 genetic risk variants for vitamin D supplementation. This could provide the first example of a genetics-based precision prevention approach.
From a research perspective, this study will provide important knowledge on the potential for improving child health through ‘prenatal programming’ during pregnancy. This is a potentially important ‘window of opportunity’, where both the immune system and respiratory organs of the fetus develop rapidly with potential long-term implications for health later in life. Furthermore, this study could reveal underlying mechanisms through exploratory studies using proteomic and metabolomic profiling of the pregnant mothers in relation to the intervention effect.
In conclusion, these large-scale RCTs, including two interventions of n-3 LCPUFA and high-dose vitamin D in pregnancy, will have the potential to change clinical guidelines in the prevention of early childhood asthma and other common disorders in early childhood.
Ethics statements
Patient consent for publication
References
Footnotes
BC and KB are joint senior authors.
X @JKyvsgaard
Contributors NB has written the first draft of the manuscript. NB, MM, NV, RV, SB, JNK, UR, JS, BLKC and KB were all involved in the design of the study, revision and final approval of the manuscript. KB is the guarantor of this study.
Funding This study received funding from Børnelungefonden (The Children’s Lung Foundation) and DFF (The Danish Independent Research Fund).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Author note The study was conducted in accordance with the guiding principles of the Declaration of Helsinki.