Article Text
Abstract
Objectives Hysterectomy or myomectomy is a common gynaecological procedure that results in moderate to severe acute postoperative pain, which can cause many adverse effects. This study aimed to compare the postoperative analgesic efficacy, opioid consumption, quality of postoperative recovery (QOR) and adverse reactions of intravenous coinjection of lidocaine and dexmedetomidine versus lidocaine or dexmedetomidine alone in gynaecological surgery.
Design Systematic review and meta-analysis was performed.
Data sources The PubMed, Embase, Cochrane Library and Web of Science databases were used to access the articles. Electronic databases were searched for eligible studies published before 1 May 2024.
Eligibility criteria All randomised controlled trials (RCTs) were included in the final analysis in which the intraoperative intervention group received intravenous coinjection of lidocaine and dexmedetomidine, and the control group received intravenous injection of lidocaine or dexmedetomidine alone in gynaecologic procedures.
Data extraction and synthesis Study retrieval, literature screening, data extraction and risk of bias assessment were performed independently by two reviewers. The quality of included studies was assessed by the Cochrane Collaboration Risk of Bias (ROB V.2.0). Data were expressed as standardised mean difference, weighted mean difference or relative risk with 95% CI. Review Manager V.5.4 was used for data analysis.
Results A total of five RCTs were included, involving 672 patients, of which 224 patients received coinjection of lidocaine and dexmedetomidine. The results revealed that coinjection of lidocaine and dexmedetomidine was superior to individual lidocaine in the visual analogue scale (VAS) scores at 1 hour (MD=−0.90, 95% CI (−1.11 to –0.69), p<0.001), 2 hours (MD=−0.99, 95% CI (−1.19 to –0.80), p<0.001), 4 hours (MD=−1.20, 95% CI (−1.75 to –0.66), p<0.001), 6 hours (MD=−1.09, 95% CI (−1.48 to –0.70), p<0.001), 8 hours (MD=−1.22, 95% CI (−1.61 to –0.83), p<0.001) and 12 hours (MD=−0.76, 95% CI (−1.35 to –0.17), p=0.o1) after surgery. Compared with the dexmedetomidine group, the lidocaine+dexmedetomidine group had low VAS scores at 1 hour (MD=−0.60, 95% CI (−0.83 to –0.37), p<0.001), 2 hours (MD=−0.70, 95% CI (−0.87 to –0.53), p<0.001), 6 hours (MD=−0.79, 95% CI (−0.98 to –0.59), p<0.001), 8 hours (MD=−0.77, 95% CI (−1.25 to –0.28), p=0.002) and 12 hours (MD=−0.56, 95% CI (−1.00 to –0.11), p=0.01) after surgery. Coinjection of lidocaine and dexmedetomidine resulted in significantly lower postoperative opioid consumption, postoperative nausea and vomiting and bradycardia than lidocaine alone (all p<0.05). Compared with the dexmedetomidine group, the lidocaine+dexmedetomidine group shortened the time to intestinal transit resumption (p=0.003). Coinjection of lidocaine and dexmedetomidine reduced intraoperative opioid consumption and increased QOR scores compared with lidocaine and dexmedetomidine alone (all p<0.05).
Conclusion Lidocaine combined with dexmedetomidine had superior analgesic efficacy and safety. However, due to the limitation in the number of available studies, more large-scale, prospective RCTs are needed for further investigation.
PROSPERO registration number
CRD42023384018.
- Drug Combinations
- Pain management
- Adult anaesthesia
- China
- Meta-Analysis
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Relevant randomised controlled trials were included in this systematic review and meta-analysis.
The well-designed search strategies without language or country restriction will offer a comprehensive search.
This paper followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines for Systematic Reviews and Meta-Analyses.
Despite conducting a comprehensive search, several of the final articles included came from the same research group, which may have introduced potential bias.
The limited data restrict the subgroup analysis conducted in this study.
Introduction
Hysterectomy or myomectomy is a common gynaecological procedure that millions of patients undergo every year worldwide according to incomplete statistics. In China, this figure is about 2.8 million cases per year.1 These gynaecological procedures can result in moderate to severe acute postoperative pain, which can cause many adverse effects, delay functional recovery, prolong hospitalisation, induce chronic pain or opioid addiction and increase medical costs.2–4 Currently, opioids are commonly used for postoperative analgesia. A large cohort study demonstrated that 86% of patients received multimodal analgesia, almost all of whom received opioid analgesics.5 Opioids are known to cause respiratory infections and depression, postoperative nausea and vomiting (PONV), pruritus, urinary retention and hyperalgesia.6–8 Therefore, the management of acute postoperative pain remains challenging and the search for suitable adjuvants to reduce opioid use is a long and arduous task.
Lidocaine is an amide local anaesthetic and class Ib antiarrhythmic drug with analgesic, antihyperalgesia, and anti-inflammatory effects and can be used as an adjuvant to general anaesthesia.9 10 Dexmedetomidine is a highly selective α2 receptor agonist with sedative, analgesic and anxiolytic effects.11 Multiple studies have noted that intravenous administration of lidocaine or dexmedetomidine can reduce early postoperative pain, promote postoperative recovery and reduce opioid consumption compared with intravenous saline administration alone.12–14 However, the postoperative analgesic effect of concomitant use of dexmedetomidine and lidocaine is unclear. Ren et al15 showed that in patients with lung cancer, there was no statistically significant difference in visual analogue scale (VAS) score and quality of postoperative recovery (QOR)-40 score 1 day after surgery between the lidocaine+dexmedetomidine group and the lidocaine or dexmedetomidine alone. Guo et al16 showed that after thyroidectomy, there was no significant difference in VAS score at 1 hour among the three groups. At 4 hours, the analgesic effect of the lidocaine+dexmedetomidine group was better than that of the dexmedetomidine group, but there was no significant difference between the lidocaine+dexmedetomidine group and the lidocaine group. However, Xu et al17 showed that VAS scores of the lidocaine+dexmedetomidine group at 1 hour, 4 hours, 8 hours, 12 hours and 24 hours after surgery were lower than those of the lidocaine group or the dexmedetomidine group. Therefore, this study aimed to conduct a systematic review of published randomised controlled trials (RCTs) to compare the postoperative analgesic efficacy, opioid consumption, QOR and side effects of intravenous coinjection of lidocaine and dexmedetomidine versus lidocaine or dexmedetomidine alone in gynaecological surgery.
Materials and methods
This paper followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines for Systematic Reviews and Meta-Analyses.18 The study protocol was registered in the International Prospective Register of Systematic Reviews.
Patient and public involvement statement
None.
Search strategy
PubMed, Embase, Cochrane Library and Web of Science were comprehensively searched using the search terms “lidocaine” AND “dexmedetomidine.” from database inception to 1 May 2024 (online supplemental table S1).
Supplemental material
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) patients: undergoing gynaecological surgery and aged over 18 years old. (2) intervention group: coinjection of dexmedetomidine and lidocaine; (3) control group: injection of dexmedetomidine or lidocaine alone; (4) outcomes: the primary outcome measure was postoperative pain scores, including the visual analogue scale (VAS). Secondary outcome measures included postoperative opioid consumption within 24 hours, the occurrence of PONV and time to intestinal transit resumption; (5) type of study: RCTs.
Exclusion criteria were as follows: (1) case reports, meta-analysis, reviews, conference papers and animal experiments; (2) republished literature; (3) articles where the full text cannot be obtained.
Literature screening and quality assessment
Two investigators independently screened the literature according to prespecified inclusion and exclusion criteria. Any disagreements were discussed or decided by a third researcher. The initially retrieved studies were imported into Endnote software to screen out duplicates. The titles and abstracts of the remaining articles were read to conduct a preliminary literature screening. The potentially eligible articles were then reviewed in full text to determine the final inclusion.
Two researchers were responsible for the assessment of the risk of bias. Any disagreements between the two researchers were resolved by discussion with a third one. Version 2 of the Cochrane risk-of-bias tool (RoB V.2) was used to assess the risk of bias for each outcome of the included RCTs through the following aspects: randomisation process, deviations from intended interventions, missing outcome data, outcome measurement and selective reporting of outcomes.19 The over risk of bias of each aspect was rated as ‘high risk’, ‘low risk’ or ‘some concerns’. The highest level of bias across the individual domains determined the overall RoB for each outcome of each included study.
Data extraction
Data were extracted into custom tables by two researchers independently. The following basic information was extracted: first author, publication year, country, study type, study group, sample size, patient age, body weight, body mass index, operation time and usage and dosage of lidocaine and dexmedetomidine. The extracted primary outcome measures included resting VAS scores at 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, 12 hours, 24 hours and 48 hours after surgery, while the recorded secondary outcomes included intraoperative and postoperative opioid consumption, the time to intestinal transit resumption, QOR score and side effects.
Data analysis
Review Manager V.5.4 was used for data analysis. Continuous data were expressed as standardised mean difference (SMD) or weighted mean difference (WMD) and 95% CI, and dichotomous data were expressed as relative risk (RR) and 95% CI. SMD was chosen when studies used different scales, measures or units, and WMD was chosen for continuous variables with the same units and measures. The heterogeneity was determined by combining I2 and p values. I2≤50% and p≥0.1 indicated no significant heterogeneity between studies, in which case a fixed-effects model was used; otherwise, there was significant heterogeneity, and a random-effects model was used. Sensitivity analyses were carried out to assess the influence of each study on the pooled results through the leave-one-out method. When the included studies ≥10, Begg’s and Egger’s tests were used to determine publication bias. P<0.05 indicated that the difference was statistically significant.
Results
Eligible studies and the characteristics
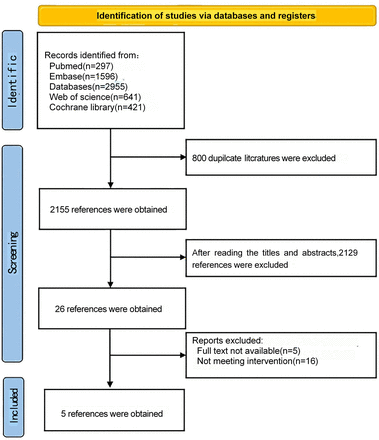
A preliminary search yielded 2955 studies, of which 2155 studies remained after the duplicates were excluded. After reading the titles and abstracts of the articles, 2129 studies were excluded, and the remaining 26 articles were included for full-text assessment. Finally, five papers were included17 20–23 (figure 1), with 672 patients, of whom 224 received coinjection of lidocaine and dexmedetomidine.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
The five included articles were published between 2017 and 2023 with a sample size of 24–60. Among them, one was on abdominal hysterectomy, three on laparoscopic hysterectomy and the other one on robotic abdominal hysterectomy. Patients who used opioids before surgery were excluded.
In the lidocaine+dexmedetomidine group, lidocaine (1.5 mg/kg) and dexmedetomidine (0.5–1 ug/kg) were administered intravenously to all patients 10–15 min before anaesthesia. The drug infusion continued until 30 min before the end of the procedure or until wound suture, with lidocaine infused at 1.5–2 mg/kg.h and dexmedetomidine at 0.4–0.5 ug/kg.h. The baseline characteristics of the included studies are displayed in table 1.
Baseline characteristics of the included studies
Quality assessment of the selected studies
The results of the RoB 2 assessment (online supplemental figure S1 and figure 2) showed that the overall risk of bias for VAS score was low. Regarding the randomisation process, all five studies were randomised using a computer random number generator, and assignments were concealed in sequentially numbered opaque envelopes containing the group allocation 1 hour before anaesthesia. There was no significant imbalance, so they were rated as ‘low risk’. Besides, all five studies were blinded to participants, intervention providers and outcome assessors, so they were rated as ‘low risk’. In terms of ‘Bias due to missing outcome data’, four studies17 21–23 had no missing outcome data and they were rated as ‘low risk’. One study20 had a balanced number of missing outcome data and the same reason for missing data between groups, as well as a small number of participants with missing outcome data, so they were also rated as ‘low risk’. As for ‘bias in outcome measurement’ and ‘bias in selective reporting of outcomes’, all five studies were rated as ‘low risk’ because they reported prespecified indicators and collected data using the same measurement method. For other outcome indicators, the risk of bias is shown in Fig 5-6, online supplemental figures S2–S5.
Overall risk of bias for VAS score. VAS, visual analogue scale.
Postoperative pain scores at rest
Coinjection of lidocaine and dexmedetomidine versus lidocaine alone
All included studies were scored by VAS and the postoperative pain scores at rest at 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, 12 hours and 24 hours were evaluated using meta-analysis. The numbers of studies and participants included at each time point in that order were 2 (n=84), 3 (n=124), 3 (n=124), 2 (n=100), 2 (n=100), 5 (n=224), 5 (n=224). In one study,23 postoperative pain scores at 1 hour, 2 hours, 12 hours and 24 hours were automatically excluded because the SD was zero. The results revealed that coinjection of lidocaine and dexmedetomidine was superior to individual lidocaine injection in the postoperative analgesic effect at 1 hour (MD=−0.90, 95% CI (−1.11 to –0.69), p<0.001), 2 hours (MD=−0.99, 95% CI (−1.19 to –0.80), p<0.001, low heterogeneity), 4 hours (MD=−1.20, 95% CI (−1.75 to –0.66), p<0.001, high heterogeneity), 6 hours (MD=−1.09, 95% CI (−1.48 to –0.70), p<0.001, high heterogeneity), 8 hours (MD=−1.22, 95% CI (−1.61 to –0.83), p<0.001, high heterogeneity) and 12 hours (MD=−0.76, 95% CI (−1.35 to –0.17), p=0.01, high heterogeneity). The pain scores of the two groups were not statistically significant at 24 hours (MD=−0.40, 95% CI (−0.95 to 0.14), p=0.15, high heterogeneity) (figure 3).
Forest plot of postoperative pain scores at rest (coinjection of lidocaine and dexmedetomidine versus lidocaine alone).
Coinjection of lidocaine and dexmedetomidine versus dexmedetomidine alone
The results indicated that coinjection of lidocaine and dexmedetomidine was superior to individual dexmedetomidine injection at 1 hour (MD=−0.60, 95% CI (−0.83 to –0.37), p<0.001), 2 hours (MD=−0.70, 95% CI (−0.87 to –0.53), p<0.001, low heterogeneity), 4 hours (MD=−0.50, 95% CI (−0.99 to –0.00), p=0.05, high heterogeneity), 6 hours (MD=−0.79, 95% CI (−0.98 to –0.59), p<0.001, low heterogeneity), 8 hours (MD=−0.77, 95% CI (−1.25 to –0.28), p=0.002, high heterogeneity) and 12 hours (MD=−0.56, 95% CI (−1.00 to –0.11), p=0.01, high heterogeneity). The pain scores of the two groups were not statistically significant at 24 hours (MD=−0.30, 95% CI (−0.79 to 0.19), p=0.23, high heterogeneity) (figure 4). In one study,23 postoperative pain scores at 1 hour, 2 hours, 12 hours and 24 hours were automatically excluded because the SD was zero.
Forest plot of postoperative pain scores at rest (coinjection of lidocaine and dexmedetomidine versus dexmedetomidine alone).
Postoperative opioid consumption
Three studies reported postoperative opioid consumption, and all patients (n=144) received patient-controlled intravenous analgesia. A random-effects model was used to analyse postoperative opioid consumption within 24 hours. Subgroup analyses showed that postoperative opioid consumption was significantly lower in the lidocaine+dexmedetomidine group than that in the lidocaine group (SMD=−4.44, 95% CI (−8.24 to –0.64), p=0.02, high heterogeneity), but no difference was found compared with the dexmedetomidine group (SMD=−2.35, 95% CI (−4.77 to –0.07), p=0.06, high heterogeneity) (figure 5).
Forest plot of postoperative opioid consumption.
Intraoperative opioid consumption
Three studies reported intraoperative opioid consumption (n=140). A random-effects model was used to analyse intraoperative opioid consumption. Subgroup analyses showed that intraoperative opioid consumption was significantly lower in the lidocaine+dexmedetomidine group than in the lidocaine (MD=−234.69, 95% CI (−355.88 to –113.49), p=0.0001, high heterogeneity) and dexmedetomidine (MD=191.01, 95% CI (−327.95 to –54.07), p=0.006, high heterogeneity) alone groups (figure 6).
Forest plot of intraoperative opioid consumption.
Postoperative nausea and vomiting
Two studies reported the incidence of PONV (n=100), and a fixed-effect model was used to analyse PONV within 24 hours after surgery. Subgroup analyses showed that there was a significant difference in the incidence of PONV between the lidocaine+dexmedetomidine group and the lidocaine group (RR=0.67, 95% CI (0.46 to 0.96), p=0.03, low heterogeneity); but there was no significant difference compared with the dexmedetomidine group (RR=0.81, 95% CI (0.55 to 1.20), p=0.29, low heterogeneity) (online supplemental figure S2).
Time to intestinal transit resumption
Three studies reported the time to intestinal transit resumption (n=124). Subgroup analyses showed no significant difference in time to intestinal transit resumption in the lidocaine+dexmedetomidine group compared with the lidocaine group (MD=−2.38, 95% CI (−8.88 to 4.12), p=0.47, high heterogeneity); but there was a significant difference compared with the dexmedetomidine group (MD=−5.71, 95% CI (−9.50 to –1.92), p=0.003) (online supplemental figure S3).
Bradycardia
Three studies reported the incidence of perioperative bradycardia (n=124). Subgroup analysis showed that the incidence of bradycardia in the lidocaine+dexmedetomidine group was significantly higher than that in the lidocaine group (RR=6.02, 95% CI (1.63 to 22.21), p=0.007, high heterogeneity), and there was no significant difference between the lidocaine+dexmedetomidine group and the dexmedetomidine group (RR=1.10, 95% CI (0.78 to 1.55), p=0.58, high heterogeneity) (online supplemental figure S4).
QOR score
Only two studies reported the QOR scores (n=80). Subgroup analysis showed that the lidocaine+dexmedetomidine group had a higher QOR score than the lidocaine (SMD=5.85, 95% CI (2.02 to 9.68), p=0.003, high heterogeneity) and dexmedetomidine (SMD=3.09, 95% CI (1.27 to 4.92), p=0.0001, high heterogeneity) alone groups (online supplemental figure S5).
Publication bias
Egger’s funnel plot was not used to assess publication bias because the number of included studies was less than 10.
Discussion
This study is the first meta-analysis to compare the effects of intravenous coinjection of lidocaine and dexmedetomidine on pain after gynaecological surgery. All related RCTs were included to support our conclusion that intravenous coinjection of lidocaine and dexmedetomidine significantly lowered pain scores in the early period (12 hours) after gynaecological surgery, reduced opioid consumption, and improved QOR compared with either lidocaine or dexmedetomidine alone.
VAS scores were used in all included studies. The results showed that postoperative pain scores at 1, 2, 6, 8 and 12 hours were significantly lower in the lidocaine+dexmedetomidine group than in the lidocaine or dexmedetomidine alone groups, suggesting that either lidocaine or dexmedetomidine alone in analgesia after gynaecological surgery is inferior to the combination. This may be related to the differences in the concomitant use of the two drugs in terms of analgesic mechanisms and control of postoperative inflammatory factors compared with their use alone.
At the level of the spinal cord, dexmedetomidine acts on the α2 receptors of the anterior and posterior synaptic membranes of the spinal cord, inhibiting the release of norepinephrine, thereby inhibiting the transmission of pain signals to the brain. At the supraspinal level, dexmedetomidine acts on the nucleus locus coeruleus of the brainstem to activate the descending medulla–spinal noradrenergic pathway, thereby inhibiting pain transmission.24 Lidocaine mainly exerts central and peripheral analgesic effects by blocking sodium ion channels.25 The analgesic efficacy of the two drugs was significantly enhanced by the combination. In addition, Xu et al found that the combined injection of lidocaine and dexmedetomidine significantly reduced plasma IL-1, IL-6 and TNF-α levels after the surgery and 2 hour postoperatively, and reduced pain at 2, 6 and 12 hours compared with dexmedetomidine injection alone.17 This suggests that the combined use of the two drugs could further control the secretion of inflammatory cytokines and reduce pain intensity compared with dexmedetomidine use alone. Furthermore, in our study, there was no significant difference in pain scores at 24 hours postoperatively in the lidocaine+dexmedetomidine group compared with the lidocaine group or dexmedetomidine group alone. This may be because the drug exerts a stable analgesic effect for 24 hours postoperatively, resulting in no significant difference in pain scores among the three groups.
Postoperative opioid consumption is also an important indicator for evaluating postoperative analgesia. The results showed that at 24 hours postoperatively, opioid consumption in the lidocaine+dexmedetomidine group was significantly lower than that in the lidocaine group. Studies have confirmed that dexmedetomidine works synergistically with opioids and that dexmedetomidine enhances the analgesic effect of opioids, thereby reducing opioid consumption.26 However, there was no significant reduction in postoperative opioid consumption in the combined group compared with the dexmedetomidine group, although postoperative opioid consumption appeared to be lower in the combined group in the three included studies. This phenomenon may be due to the small sample size of the three included studies.
Three studies used remifentanil for both induction and maintenance of anaesthesia and reported the total amount of remifentanil used. The results showed that intravenous coinjection of lidocaine and dexmedetomidine significantly reduced intraoperative remifentanil use compared with lidocaine and dexmedetomidine alone. Lidocaine and dexmedetomidine exert their analgesic effects through different mechanisms and reduce the anti-injury response, thus reducing intraoperative remifentanil use. There may be a superimposed or synergistic effect of lidocaine and dexmedetomidine. However, the wide CIs for the combined results may be due to the high internal variability of the data resulting from different surgical procedures, and thus their accuracy should be interpreted with more cautions.
PONV is the second most common complication after postoperative pain, with an overall incidence of approximately 30%, and women, non-smokers and opioid use are all risk factors.27 A meta-analysis28 revealed that dexmedetomidine reduced the incidence of PONV by inhibiting the release of catecholamine and opioid retention through parasympathetic tone. Ahn et al29 showed that intravenous infusion of lidocaine in laparoscopic colon surgery could reduce the incidence of postoperative nausea. Our study noted a significant difference in the incidence of PONV in the lidocaine+dexmedetomidine group compared with the lidocaine group, but no significant difference was found when compared with dexmedetomidine. It may be related to the fact that the combination significantly reduces postoperative pain and decreases the use of intraoperative and postoperative opioids, which in turn mitigates complications such as PONV and slowed bowel movements.
Our study found that coinjection of lidocaine and dexmedetomidine significantly reduced the time to intestinal peristalsis compared with the lidocaine alone group, but no significant difference was found when compared with dexmedetomidine alone. Opioid consumption could directly affect enteric opioid receptors, which further delays the return of bowel activity.30 This may explain why the postoperative time to recovery of bowel function was shorter in the combination group than in the lidocaine group. Opioid consumption was significantly reduced in the combination group compared with the lidocaine group, both intraoperatively and postoperatively. Besides, several studies have shown that dexmedetomidine inhibits gastric emptying and promotes the recovery of gastrointestinal function.31–33 In addition, these results should be viewed with caution due to the small sample size of included studies, and more studies are expected to be included in the future.
Dexmedetomidine infusion causes bradycardia, hypotension, arrhythmias and prolonged sedation. Unfortunately, hypertension and arrhythmias were not reported in the included studies, and only one study20 reported the number of patients with a Ramsay sedation score of ≥4 in the PACU. The combination groups did have more patients with a Ramsay sedation score of ≥4 than the lidocaine group. Three studies reported the incidence of bradycardia, with a significantly increased incidence of bradycardia in the combination group compared with the lidocaine group. On the one hand, the heart rate is slowed down due to dexmedetomidine’s activation of cardiac beta receptors, and on the other hand, the heart rate is affected due to dexmedetomidine’s activation of alpha-2 adrenergic receptors, which dilate blood vessels.11
The QOR is a widely used global scale for measuring quality of recovery, of which the QOR-15 is a shortened version of the QOR-40. The intravenous coinjection of lidocaine and dexmedetomidine further improved the QOR than lidocaine and dexmedetomidine alone. This improvement may be related to better postoperative pain relief, reduced opioid consumption, lower incidence of PONV and promotion of bowel recovery in the combined group.
Notably, there was high heterogeneity in some of the results, possibly due to differences in surgical procedures, subjective measurements or small sample sizes. This may reduce the reliability and stability of the combined results. More large-sample, multicentre clinical studies are expected in the future.
Unfortunately, four included studies were from the same research group, which is indeed a limitation of our work. Currently, research on intravenous coinjection lidocaine and dexmedetomidine is limited. Foreign researchers are mainly concerned about the efficacy of opioid-free anaesthesia in various surgical procedures. Opioid-free anaesthesia may not provide the same analgesic effect as opioids. In addition, opioid-free anaesthesia requires experience and skills. Notably, opioid-free anaesthesia will use more other drugs, which undoubtedly increases the risk of side effects from these drugs. Therefore, most studies we included focused on reducing opioid consumption, as included studies were mainly from China. These studies used similar experimental designs with samples from the same region, limiting the generalisability of the findings. Future studies should consider more different study groups, different regions and different types of surgeries, thus improving the reliability and applicability of the findings. Besides, there are some other limitations to our study. First, only 5 RCTs with 672 patients were included. Thus, the conclusions need to be interpreted with caution. More high-quality RCTs are warranted. Second, most included study populations were Asians, which may limit the results due to racial differences in drug metabolism. Finally, subgroup analyses were not conducted in this meta-analysis due to limited studies.
Conclusions
In conclusion, the intravenous coinjection of lidocaine and dexmedetomidine in gynaecological surgery is associated with lower postoperative pain, reduced intraoperative opioid use and improved QOR compared with either lidocaine or dexmedetomidine alone. In addition, intravenous coinjection of lidocaine and dexmedetomidine significantly reduces the incidence of PONV and postoperative opioid consumption but increases the incidence of bradycardia compared with lidocaine alone, and the combination group significantly shortens the time to intestinal transit resumption compared with dexmedetomidine alone. However, there was significant heterogeneity among the different included studies. Due to the insufficient number of studies, subgroup analyses could not be performed to look for potential sources of heterogeneity. Therefore, more high-quality clinical studies are needed in the future to determine the analgesic effects of intravenous coinjection of lidocaine and dexmedetomidine.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Footnotes
Contributors All authors contributed to the study conception and design. Writing—original draft preparation: DX, FW; writing—review and editing: DX, WW, HL; conceptualisation: DX; methodology: DX, FW, WW; formal analysis and investigation: FW, WW, HL; resources: DX; supervision: DX; and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Guarantor: DX.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer-reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.