Article Text
Abstract
Introduction Despite the rapid advancements in cardiovascular surgery in China, the prevalence of valvular heart disease (VHD) continues to rise, particularly among the elderly population. In the resource-constrained western regions, the lack of an integrated care management system significantly contributes to the burden of cardiovascular disease. Consequently, a comprehensive cohort data platform that encompasses the entire lifespan of patients with VHD is essential. This prospective cohort study aims to facilitate the examination of risk factor screening, disease progression, diagnostic and treatment strategies, and the long-term functional recovery trajectories of patients following valve surgery.
Methods and analysis The Integrated Whole-Life Cycle Accuracy Valvular Heart Disease Epidemiology Cohort Study is a prospective cohort study that plans to enrol approximately 10 000 participants, including both patients with VHD and members of the general population, by 2028. Led by the West China Hospital of Sichuan University, it will be conducted in collaboration with 15 medical consortiums and their affiliated community hospitals. This study seeks to assess the disease trajectory of VHD, as well as the risk factors and protective measures that influence its progression and prognosis. This study will collect and analyse basic demographic information, peripheral blood and tissue samples, long-term functional follow-up data, and patient-reported outcome questionnaires. Additionally, electronic health records will be used to document patients with VHD undergoing surgical interventions, along with lifetime endpoint events for the valve clinical study.
Ethics and dissemination The study protocol was approved by the Biomedical Research Ethics Committee of West China Hospital of Sichuan University (No. 20232422). All participants will be required to provide written informed consent. The study findings will be disseminated via publications in peer-reviewed journals and presentations at scientific conferences.
- Valvular heart disease
- Patient Reported Outcome Measures
- Quality of Life
- Observational Study
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The Integrated Whole-Life Cycle Accuracy Valvular Heart Disease Epidemiology Cohort Study will use an established integrated care platform and a well-designed electronic data capture system, facilitating effective disease registration and continuous tracking, which will support high follow-up rates.
This study will monitor patients’ progression from high-risk factors to a confirmed diagnosis of valvular heart disease (VHD), including surgical treatment and ongoing follow-up until an endpoint event occurs, thereby providing valuable longitudinal data to enhance the understanding of the development of VHD.
The cohort represents only the VHD population in western China and may not adequately reflect the characteristics of VHD across the entire Chinese population.
The study design limits the analysis of correlations between various medical decisions and long-term patient prognosis; however, incorporating a multicentre cohort may provide a sufficiently large sample size for propensity score-matched analyses to investigate these correlations.
Introduction
Valvular heart disease (VHD), which primarily results from rheumatic heart disease or degenerative changes, represents an increasing public health concern. Approximately 25 million individuals in China currently experience VHD, with 55.1% affected by rheumatic VHD and 21.3% affected by degenerative VHD.1 In recent years, cardiac surgery in China has advanced rapidly, marked by a significant increase in the number of procedures performed.2 However, notable economic and educational disparities persist across various regions of the country, resulting in considerable fluctuations in the quality of cardiac surgeries.3 Unlike the more economically developed eastern regions, which benefit from efficient health resource allocation, the middle-income to low-income western regions endure a significant burden of cardiovascular disease because of insufficient health resources and limited access to high-quality healthcare services. Consequently, the burden of cardiovascular disease in western China has increased dramatically over the past decade. 1 4
One reason for the poor survival of patients with VHD in China is the lack of early echocardiographic screening and diagnosis among asymptomatic individuals, which indirectly leads to delayed consultations. 5 A recent survey indicated that a significant majority of elderly patients with VHD hospitalised in China present with evident symptoms (89.4%); however, only 37% undergo surgical interventions, often due to personal refusal, disease severity and high surgical risk.6 Early detection, rational assessment and optimal referral are crucial for improving VHD outcomes. Therefore, early disease screening and the establishment of medical information registries are necessary for community populations in western China, where medical resources are limited. Moreover, an increasing number of patients are undergoing cardiac surgery. Although several clinical trials and observational studies have demonstrated that postoperative heart function improves over time,7 postoperative functional decline remains common among elderly patients due to comorbidities and frailty.8 Successful surgery initiates a lifelong journey that may involve potential adverse outcomes, including prosthetic valve failure, stroke and heart failure re-admission. Regular appointments with a cardiologist are essential, as clinical deterioration can occur unnoticed.9 Quality of life and functional capacity outcomes are increasingly recognised as important indicators of cardiac surgery success. Analysing functional trajectories through generic measures of functional status can yield valuable insights.10
Cohort databases significantly enhance our understanding of cardiovascular surgery and play a crucial role in advancing clinical management and patient care.11 Notable examples include the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (ACSD),12 established in 1989, which encompasses over five million cardiac surgery patients, and the Europe-wide Adult Cardiac Surgery Practice Information Database, initiated in 2002 by the European Association for Cardiothoracic Surgery.13 In 2013, the Chinese National Center for Cardiovascular Disease and Fu Wai Hospital launched the Chinese Cardiac Surgery Registry.14 However, these databases predominantly focus on patients with moderate-to-severe VHD, often overlooking risk factors and epidemiological studies concerning patients with asymptomatic and mild VHD.15 Furthermore, many cohort databases lack comprehensive data on long-term survival and quality-of-life improvements.16 The Chinese healthcare system is primarily oriented towards diagnostics and surgical interventions,17 with less emphasis on primary care and disease management, which are vital for enhancing long-term patient outcomes.18 Consequently, establishing an early screening and lifelong follow-up system tailored to the characteristics and treatment needs of the Chinese VHD population is essential for improving long-term health outcomes.19
Western China, which encompasses 12 provinces and covers 6.87 million square kilometres, including Sichuan Province, exhibits considerable diversity, with unique geographical and climatic conditions, and hosts 56 ethnic minorities. This region, which represents 27.2% of China’s population, has significant genetic diversity and distinct lifestyle choices, such as the Tibetans’ adaptation to low oxygen levels on the plateau and the Miao people’s sour soup-rich diet.20 Established in 2009, the West China Hospital Biospecimen Bank of Sichuan University has the capacity to store 10 million samples, supporting biospecimen collection, epidemiological investigations, and follow-ups for natural populations and various disease cohorts. The Integrated Whole-Life Cycle Accuracy Valvular Heart Disease Epidemiology (iWAVE) Study project, which is being operated by the National Clinical Research Center for Geriatrics at West China Hospital, Sichuan University, is leveraging the existing integrated care management system and collaborating with 15 medical consortiums and their affiliated community hospitals in the region to develop a cohort database focused on the whole-life cycle management of patients with VHD.19 As China’s ageing population increases—projected to reach 395 million individuals aged 65 and older by 2050—the data gathered from this cohort will improve our understanding of VHD characteristics in Western China and facilitate the establishment of localised standards for early diagnosis, treatment and rehabilitation.21 Our goal is to implement a patient-centred approach that encompasses health promotion, disease screening, prevention, diagnosis, treatment and rehabilitation, ultimately increasing patients' quality of life. To achieve this goal, we will collect prospective data, including cardiopulmonary exercise assessments; peripheral blood, myocardial and valve tissue samples; medical test results; imaging data; and electronic health records, and we will perform long-term functional follow-ups to evaluate cardiac structure and function.
Aims
The outcomes and long-term functional recovery trajectories of patients with VHD across multiple dimensions, including age, disease severity and surgical modality, will be collected, and the risk and protective factors that influence individual outcomes throughout the disease lifespan will be identified.
Postoperative clinical outcomes of patients with VHD, such as adverse events during hospitalisation, long-term follow-up outcomes and duration of hospital stay, will be analysed to provide a comprehensive understanding of postoperative results and identify predictive factors.
Mortality prediction tools adapted specifically for short-term and long-term outcomes in patients with VHD within the Chinese population will be developed, and these tools will be compared with the STS score calculator.
Personalised whole-life cycle follow-up profiles will be established on the basis of patients’ clinical characteristics, the implementation and efficacy of an integrated care management system for VHD will be assessed, and the role of a specialist disease manager in promoting long-term functional recovery will be evaluated.
Epidemiological data on VHD prevalence within a natural population cohort in Western China will be collected, and the impacts of socioeconomic, psychological and physiological factors, along with activity levels and living environments, will be assessed.
The trajectory of VHD development within a natural population cohort will be explored by using peripheral blood or tissue samples to identify critical periods of ventricular remodelling and optimal intervention windows.
Methods and analysis
Study design and setting
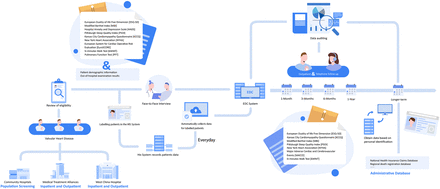
iWAVE is a prospective, multicentre, observational, population-based cohort study initiated at West China Hospital, West China Tianfu Hospital, and Shang Jin Hospital of West China Hospital. The project will progressively expand to include the 15 hospitals within the West China Hospital Medical Consortium and their affiliated community hospitals for ongoing participant recruitment (figure 1). The National Clinical Medical Research Center for Geriatric Diseases and the Heart Valve Disease Research Center at Sichuan University’s West China Hospital will manage the study’s design and implementation.
Distribution of West China Hospital and units of the 15 participating medical consortiums.
Study population
The categorisation of participants on the basis of the source population is detailed below. (1) Community-based natural population cohort: Eligible participants are permanent residents within the jurisdiction of the West China Hospital Medical Consortium who agree to undergo transthoracic echocardiography at community hospitals. Sonographers conducting the procedures will receive standardised training from the Departments of Ultrasound and Cardiology at West China Hospital. The inclusion criterion is that the participants must be 35 years of age or older. The exclusion criteria include an inability to comprehend the questionnaire or refusal to participate in the study. (2) Inpatient and outpatient follow-up cohort: This group consists of participants from the cardiovascular surgery departments of West China Hospital and 15 associated medical consortiums. The eligibility criteria include patients initially evaluated with moderate or more severe VHD via echocardiography, being 35 years of age or older, and having the capacity to understand the questionnaire. The exclusion criteria include impaired consciousness or death that prevents the completion of the questionnaire, a life expectancy of less than 6 months, acute aortic dissection and refusal to participate in the study.
Study procedure
The data elements and definitions for the iWAVE Cohort Study form were meticulously designed, drawing inspiration from the current STS ACSD database template and adapting it to the specific needs of this study. Figures 2 and 3 provide detailed information on the study process.
Recruitment process of the Integrated Whole-Life Cycle Accuracy Valvular Heart Disease Epidemiology (iWAVE) Cohort Study. EDC, electronic data capture.
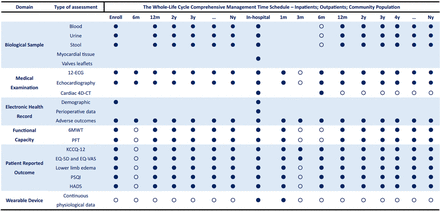
Summary of clinical data collection.ECG:Electrocardiogram; 4D-CT: 4-Dimensional Computed Tomography; 6MWT: 6-minute Walking Test; PFT: Pulmonary Function Test; KCCQ: The Kansas City Cardiomyopathy Qusetionnaire; EQ-5D: EuroQol Five Dimension Scale; EQ-VAS: EuroQol Visual Analogue Scale; PSQI: Pittsburg Sleep Quality Index; HADS: Hospital Anxiety and Depression Scale. Mandatory;
Mandatory;  Available.
Available.
To streamline data collection and organisation, we developed a Chinese electronic data capture (EDC) system. This system requires network connectivity via a computer or handheld mobile device, with each hospital site authorised to access the EDC system via an assigned account. The system accommodates various unit levels, including community hospitals within medical unions, medical union units and the central hospitals of West China Hospital, Sichuan University. To address the unique characteristics of patient diagnosis and treatment across different levels in the western region of China, specific electronic forms within the EDC system will be designated for data collection at each unit level. To comprehensively delineate the characteristics of patients with VHD, community hospitals affiliated with the medical consortium will primarily gather community-based health examination data from patients. This information will facilitate patient screening and categorisation, guiding regular follow-ups within the community on the basis of disease severity. In contrast, the medical consortium and West China Hospital, Sichuan University, will primarily collect diagnostic and treatment data during hospital visits. The integrated care management system will include patient follow-up components with scheduled follow-ups at 1 month, 3 months, 6 months and 1 year postdischarge, followed by regular annual outpatient follow-ups, addressing instances involving adverse events that require medical attention.
Trained VHD data collectors will conduct daily eligibility screenings for patients in outpatient clinics and hospital wards. On identifying eligible patients, the EDC system will automatically compile their essential information. This includes the patient registration number, which serves as a unique identifier within the treating hospital; the outpatient visit number for the current visit; the inpatient case number corresponding to the ongoing hospitalisation; the patient’s ID card number as a distinctive patient identifier; and the phone number as the primary contact method. Within the EDC system, a new account is established for each eligible patient, with these details recorded as essential information. When new VHD patient data are entered into the EDC system, a unique index identifier is automatically generated for the patient. The system then correlates the patient’s medical records within the medical consortium units on the basis of the patient’s ID card number. It autonomously collects patient treatment data using the registration number, visit number or case number, thereby constructing comprehensive medical records within the medical consortium.
Continuous follow-up of patients is a crucial aspect of this study, addressing the scarcity of long-term follow-up data for patients with VHD both domestically and internationally. Trained physicians will conduct ongoing follow-ups and gather patient-related information through face‒to‒face interviews via touch screen questionnaires or paper records. Full-time data collectors execute the follow-up data collection, meticulously verifying and standardising questionnaire responses before entering them into the EDC system to ensure accuracy and consistency. The EDC system dispatches follow-up reminders to data collectors 1 week before each scheduled follow-up (at 1 month, 3 months, 6 months and 1 year postdischarge, among others). We will make three attempts to contact patients on different dates and times, depending on the availability of the contact person, as indicated by phone records. All subsequent calls will be recorded for verification, and regular quality control assessments will be conducted on the follow-up results. In cases where patients cannot be contacted, we plan to undertake additional steps to obtain long-term health-related outcomes on the basis of personal information. This involves correlating the patient’s ID card number with data from the Chinese Center for Disease Control and Prevention’s national disease surveillance system, the cause of death surveillance network report database, the inpatient data identifier from the community hospital in the patient’s residential area, and regional death registration records. If these follow-up methods are unsuccessful, the case will be categorised as a loss to follow-up. Notably, participants who are unable to complete follow-up procedures will still be contacted for subsequent follow-ups unless they actively request the cessation of communication. The data collected during follow-up can be linked with patient records in the EDC system using the patient’s ID card number as the matching identifier. Ultimately, the patient’s timeline for medical and outpatient follow-ups can be organised according to treatment and follow-up dates.
To ensure the scientific rigour of the study, a consulting committee comprising experts in cardiac surgery, interventional cardiology, advanced imaging cardiology, echocardiography, heart failure, critical care, cardiac anaesthesia, cardiorespiratory physiotherapy, specialist disease management, statistics, computer engineers and other specialised fields was established. Additionally, a project management and coordination centre was created to oversee and support the iWAVE operation, conduct data quality control, and facilitate regular online and offline data quality control meetings. An independent supervisory committee was also appointed to oversee the entire research process.
Outcomes
Primary outcomes
The long-term goal of this research project is to explore the full life cycle process of natural populations as they progress from disease risk factors to VHD, undergo surgical treatment and continue through lifelong follow-up. Primary outcomes will be measured in two cohorts:
Inpatient and outpatient follow-up cohort: This cohort aims to determine the long-term (5 years and beyond) mortality rate of patients with VHD who undergo surgical interventions and are enrolled in the integrated care management system.
Community-based natural population cohort: This cohort will investigate the incidence of VHD within a natural population in western China.
Secondary outcomes
The secondary endpoints of this research project are observational, and an initial exploratory study will be conducted to investigate the following:
Explore and identify multiomics predictors (including genomic and miRNA signatures, as well as specific proteins) for the development of left ventricular remodelling in patients with VHD.
To determine the incidence of short-term (1-month, 3-month and 6-month) and long-term (more than 1 year) adverse events in patients undergoing surgery on the basis of the ‘Valve Academic Research Consortium 3 (VARC-3) endpoint events’ and to identify the corresponding protective factors and risk factors.
To assess the long-term functional trajectories of patients with VHD by analysing changes in patient self-reported outcomes and functional capacity measures.
Data collection
The following data will be collected for all participants in both the inpatient and outpatient follow-up cohorts, as well as the community-based natural population cohort:
Basic demographic information and peripheral blood samples.
Full life cycle medical imaging data.
Long-term functional follow-up and patient self-reported outcome questionnaires.
Inpatient and outpatient follow-up cohorts:
i. Peripheral venous blood parameters, including cardiac biomarkers, biochemical indices, coagulation, routine blood cell analyses, endocrine hormone measurements, glycosylated haemoglobin levels, inflammatory factors, and samples for cell-free DNA analysis, miRNA analysis, cytokines, telomere length, metabolomics, proteomics, transcriptomics, and genomics.
ii. Routine biochemical indicators of urine and stool and samples for precision medicine tests, such as microbial sequencing.
iii. Myocardial and/or valvular tissue samples.
iv. Blood pressure, 12-lead ECG and peripheral oxygen saturation.
v. Echocardiogram and Doppler measurements and cardiac 4-dimensional CT (4D-CT) measurements.
vi. Electronic health record information, including comorbidity information, medication use, surgical risk assessment, surgical operative records, anaesthesia monitoring data, critical care records and in-hospital adverse outcome events.
vii. Valve clinical study endpoint events tracked throughout the patient’s lifetime.
viii. Functional capacity measures, including the 6 min walk test (6MWT), pulmonary function test (PFT) and New York Heart Association (NYHA) classification.
ix. Patient self-reported outcome questionnaires: The Kansas City Cardiomyopathy Questionnaire-12 (KCCQ-12) Questionnaire; EuroQol Five Dimension Scale (EQ-5D) and EuroQol Visual Analogue Scale Questionnaires; lower extremity oedema level; Pittsburgh Sleep Quality Index (PSQI); General Anxiety Disorder Scale-7 (GAD-7); Patient Health Questionnaire-9 (PHQ-9).
x. Home wearable devices to capture continuous physiological parameters.
Community-based natural population cohorts:
i. Peripheral venous blood analysis, routine biochemical indicators in urine and stool, and sample collection for precision medical testing.
ii. Blood pressure, 12-lead ECG and peripheral oxygen saturation.
iii. Echocardiograms and Doppler measurements.
iv. Electronic health record information, including comorbidity information and medication utilisation.
v. Major adverse cardiovascular events tracked throughout the patient’s lifetime.
vi. Functional capacity measures, including the 6MWT, PFT and NYHA classification.
vii. Patient self-reported outcome questionnaires.
Venous blood, urine and stool sampling
Initial peripheral venous blood, urine and stool samples will be collected when patients provide informed consent and enrol in the cohort study. For the inpatient and outpatient follow-up cohorts, additional peripheral blood samples will be obtained on the day of discharge. Subsequent routine outpatient follow-up visits will be conducted at least once a year at the hospital where the procedure was performed, allowing for the collection of peripheral venous blood, urine and stool samples. For the community-based natural population cohort, routine physical examination screenings will be performed at least annually at the community hospital to collect peripheral venous blood, urine and stool samples.
Myocardial and/or valve tissue sample collection
This programme is designed for patients undergoing heart valve surgery, either via median sternotomy or transapical catheter valve surgery. Samples of localised myocardial tissue (including the left atrium, left ventricular myocardium and right atrium) and/or valvular tissue (including the aortic, mitral and tricuspid valve leaflets) will be collected without damaging normal tissues. Additionally, whole blood, serum, plasma, faecal matter, urine and other tissue samples will be stored in a biobank for future studies.
Echocardiography and Doppler measurements
Experienced echocardiographers will assess the extent of a patient’s valvular disease and quantify their cardiac structural and haemodynamic parameters according to the guidelines from the American Society of Echocardiography for the Evaluation of Native Valvular Regurgitation5 and Rheumatic Heart Disease,22 as well as the joint guidelines from the European Association of Cardiovascular Imaging and the American Society of Echocardiography Assessment of Aortic Valve Stenosis.23
Cardiac 4D-CT examination and measurements
Patients scheduled for transcatheter aortic valve implantation (TAVI) will undergo CT data acquisition and reconstruction in accordance with the TAVI Expert Consensus Document of the Society of Cardiovascular CT.24 This examination assesses dynamic changes in aortic root geometry and dimensions while providing anatomical information about the vascular system, including the aorta, iliac arteries and femoral arteries.
Electronic health record information—in-hospital adverse outcome events
The iWAVE Study project framework selected postoperative outcome events that align with those in prominent international databases, such as the STS ASCD.25 These events include operative mortality, stroke, renal failure, prolonged ventilation, reoperation, composite morbidity and mortality, prolonged postoperative length of stay, short postoperative length of stay, and deep sternal wound infection. Although the database primarily represents the US population, which significantly differs from the target population of this study, it is crucial to maintain consistent definitions of outcome events. This consistency will facilitate future population-specific analyses, comparisons of VHD management techniques and concepts,26 and the development of risk models tailored to the Chinese VHD population.
Valve clinical study endpoint events tracked throughout the patient’s lifetime:
The iWAVE Study project selected clinical research endpoints developed by VARC-3.27 These endpoints include mortality, neurological events, hospitalisation (or rehospitalisation), bleeding and transfusions, vascular and access-related complications, cardiac structural complications, other procedural or valve-related complications, new conduction disturbances and arrhythmias, acute kidney injury, myocardial infarction, bioprosthetic valve dysfunction, leaflet thickening and reduced motion, clinically significant valve thrombosis, patient-reported outcomes and health status, and composite endpoints. The endpoints and criteria established by the VARC will ensure consistent reporting, adjudication, and comparison of results across valve devices and treatment strategies.
Six-min walk test (6MWT)
The 6MWT is a submaximal exercise assessment tool widely used to evaluate cardiopulmonary function and prognosis in patients, with the 6-min walk distance serving as the primary outcome indicator.28 Patients perform the 6MWT according to the standardised protocol outlined in the American Thoracic Society guidelines under the supervision of a cardiopulmonary physical therapist.29 The test is terminated if any of the following symptoms occur: chest pain, loss of consciousness, intolerable dyspnoea, falling, profuse sweating or pallor.
Pulmonary function test (PFT)
A cardiopulmonary physical therapist will conduct PFT using the standard protocols established by the American Thoracic Society and the European Respiratory Society Technical Statement.30 These tests evaluate the effects of VHD on pulmonary function and airway responsiveness. The patient is positioned appropriately for spirometry, and the test is repeated as needed. The test is terminated if the patient experiences severe dyspnoea, marked panic, chest tightness, precordial pain, dizziness, cyanosis or pallor. The recorded indicators include forced vital capacity, forced expiratory volume in 1 s and maximum voluntary ventilation per minute.
Patient self-reported outcomes
Patient heart failure symptoms will be assessed using the KCCQ-12, the most commonly used tool for evaluating heart failure symptoms.31 Patients rate the impact of their heart failure symptoms on 12 items, including mood, daily activities and sleep.
To evaluate patients’ health status, the standardised health measurement tool EQ-5D will be employed.32 Widely used globally to determine quality of life, the EQ-5D comprises a descriptive system and predetermined utility values.33 The descriptive system includes five dimensions, namely, daily activities, self-care, mobility, anxiety/depression and pain/discomfort, each with five severity levels: extremely, severely, moderately, slightly and none. A health utility calculator for the Chinese population, available online (www.valueinhealthjournal.com/issues), provides health utility status values ranging from 0 (dead) to 1 (perfectly healthy).
To assess oedema, pressure will be applied with the thumb for at least 2 s over the dorsum of the foot, behind the medial malleolus and on the lower calf above the medial malleolus. The depth of the indentation and the time required for the skin to return to its original state will be recorded and rated on a clinical scale. Patient sleep quality will be evaluated using the PSQI, which consists of 19 items that assess subjective sleep quality, time to fall asleep, sleep duration, habitual sleep efficiency, sleep disturbances and the use of sleeping medication.34 To assess anxiety and depression, the GAD-7 and PHQ-9 will be used to monitor the severity of illness.35 36
Home wearable device to collect continuous dynamic physiological parameters
A medical-grade wearable device, developed in collaboration with an engineering team, will continuously and accurately capture dynamic physiological data before, during and after a patient’s walking activity. The physiological data obtained from the wearable device are temporally divided into three segments: the preactivity resting phase, the activity phase and the postactivity recovery phase. Automatically calculated according to predefined data preprocessing rules, the parameters include: (1) ECG metrics such as heart rate, duration of each waveform, ST segment changes, rhythm and heart rate variability; (2) Oxygen saturation characteristics; (3) Triaxial acceleration metrics, including the mean, SD, extreme values, root mean square, skewness, kurtosis, gait cycle, activity recognition features, exercise intensity, inclination, and synthetic acceleration; and (4) Adverse events during walking, such as intolerable dyspnoea, palpitations, dizziness, chest pain or tightness, fatigue, and hypoxia, along with the total walking distance.37 The ability of wearable devices to capture high-frequency, continuous physiological parameters presents significant potential for personalised health assessment and early warning systems for postoperative patients at home through artificial intelligence (AI)-driven analysis. Designed for remote monitoring, the device facilitates personalised health assessments and early interventions on the basis of continuous data collection and AI analytics.38
Sample size
On the basis of the literature review and the research team’s preliminary retrospective data, the 5-year average survival rate for patients with VHD managed via conventional protocols and who are undergoing surgical interventions is approximately 60%.10 We anticipate a 30% improvement in this survival rate with the implementation of a novel integrated care management protocol for patients with VHD. With a superiority ratio of 1.2, a test level of 0.025, a test efficacy of 0.90 and a de-escalation rate of 20%, at least 1889 VHD inpatients and outpatients need to be enrolled. Furthermore, this study aims to investigate the prevalence of VHD within the broader community. According to a survey, the weighted prevalence of VHD among the Chinese population aged 35 years and older is 3.8%.1 The likelihood of the sample rate obtained during the formal survey differing from the known prevalence by no more than 1% does not exceed 0.05. Considering a deleterious rate of 20%, a minimum of 7270 patients from the community-based population is required.
The West China Hospital of Sichuan University is one of the largest tertiary hospitals in western China, serving a population that constitutes approximately 6% of the country’s total population within its 15 medical consortiums and associated subcommunity hospitals.39 Therefore, we plan to enrol at least 10 000 patients over a 5-year period (1 October 2023 to 1 October 2028) to achieve the objectives of this prospective cohort study.
Data management and monitoring
The information stored in the iWAVE Registry Database is unalterable and can only be accessed for query analysis through database views. The EDC system provides standardised forms for VHD follow-ups and is available in both mobile and personal computer versions. Trained data collectors will be authorised to conduct follow-ups and collect data exclusively within the hospital’s internal network environment, using tablets or physician workstations. Data integration will occur through a dedicated network channel in the medical cloud to ensure data security.
All collected data will undergo daily synchronisation, where information from the hospital’s electronic health record system will be automatically integrated with the EDC system. This synchronisation will retrieve comprehensive data about participants' medical management, laboratory test results and, if applicable, surgical procedures throughout the research period. To establish a connection between health records in a fully covered participant management database for long-term medical or mortality records 1 year after recruitment and beyond, an annual linkage will be implemented (figure 2).
The EDC system data collection form template was developed by the Heart Valve Team at West China Hospital, Sichuan University. All units within the medical consortium, including community hospitals, will adhere to this standardised template for data collection. Before data collection begins, researchers and data collectors will undergo comprehensive training covering key elements, definitions and the data input system. The Project Management Coordination Center will conduct regular meetings to provide feedback on data quality control reports and implement retraining as needed. Additionally, there will be a strong emphasis on maintaining the stability of the onsite team members. When there are changes in onsite researchers, coordinators or data collectors, prompt training for new staff becomes imperative.
Participating sites will ensure the reporting of all eligible cases to iWAVE, with the specific methods outlined below. For sites using electronic medical records, an electronic query algorithm will be employed to identify patients with VHD by searching for data fields that are most likely to indicate relevant events among all patient records involved in cardiovascular surgery. These events may include conditions such as ‘rheumatic heart disease’, ‘degenerative heart disease’, ‘valvular stenosis’ and ‘valvular regurgitation’. Coordinators will subsequently evaluate compliance with the inclusion criteria on the basis of the electronic medical records. In cases where electronic medical records are not used or lack statistical functionality, such as when patients present external examination reports or hospital records, the coordinators will manually review the patients’ external paper records. The accuracy and completeness of the reported data will be ensured through a multifaceted approach. First, the EDC system incorporates data input checks to mitigate errors and enhance the precision of data collection. An algorithmic quality check system automatically prompts notifications for researchers to rectify or review data deviations from predefined rules. Second, quality inspectors will meticulously examine each variable on the basis of medical records within 7 days of data submission. If incorrect, suspicious or incomplete data are identified during ongoing audits, researchers reporting on patients with VHD must correct or complete the records and clarify any missing or dubious data elements. The inspectors will summarise the monthly progress of data collection and quality measures and engage in regular discussions with the principal investigator. Third, statisticians will routinely evaluate and screen the collected data, including checks for logic errors and outliers. Fourth, the project management centre will conduct random testing on 10%–50% of the data weekly to examine extreme values or missing entries. Fifth, the project management centre will deploy a coordinator to hospital wards and outpatient clinics every 3–6 months for onsite checks, ensuring the continuity, completeness, and accuracy of the reported data. Finally, the experts will randomly review more than 20% of the audio recordings from the entire interview process to ensure the quality of the baseline and subsequent data. In cases of disagreement, the data collectors and quality inspectors will collaboratively reassess the data (figure 4).
Data management and quality control. EDC, electronic data capture.
Data security
The EDC system allows access exclusively within the confines of the hospital’s internal network, with access permissions tailored to the specific responsibilities of individual researchers. Each researcher is assigned a unique account, and user authentication is achieved through secure passwords, thereby reducing the risk of unauthorised access to the EDC system. In accordance with ethical standards, the EDC system employs anonymisation and deidentification processes for reported data. All data transfers are securely conducted within encrypted tunnels and stored in the database of West China Hospital, Sichuan University Information Center. To minimise the risk of data loss, routine backups are systematically performed for all storage.
Statistical analysis
Data will be presented as percentages for categorical variables and as the means±SDs or medians (25th to 75th percentiles) for continuous variables. The distribution of continuous variables will be examined via the Kolmogorov‒Smirnov test. Comparisons will be made using Student’s t-test or the Mann‒Whitney U test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables, as appropriate. Linear mixed-effects models will be employed to assess changes in clinical parameters (eg, echocardiographic measurements, indicators of functional capacity) from the time of surgical intervention to long-term follow-up.
Survival analyses will be performed using the Kaplan‒Meier method to report annual event rates. Model survival functions will be estimated using risk ratios associated with predetermined key covariates adjusted for age, sex and other potential confounders, including comorbidities and relevant echocardiographic characteristics. The influence of prognostic factors on patients with VHD will be explored through multivariate Cox proportional hazards regression modelling, with the degree of influence quantified via HRs with 95% CIs. The selection of variables for the model will be based on their clinical importance and will include process variables such as VHD-specific characteristics, preoperative assessments, surgery-related information and intensive care management. Independent predictors will be selected using Stata’s Lasso technique with cross-validation.
Binary logistic regression, which is based on L1 and L2 regularisation, will be applied with the occurrence of the outcome event as the dependent variable. The coefficients from the logistic regression model rank the importance of each parameter, constructing a feature screening model on the basis of the importance of the parameters and the model’s predictive power. The best subset of features will be determined by iterating through the extracted features for selection until stability in the feature set is achieved. Fivefold cross-validation will be used to generate the training and validation sets, where the training set builds the model and the validation set confirms the model’s capabilities. We will experiment with the selected features using various machine learning models, including logistic regression, support vector machine, linear discriminant analysis, k-nearest neighbours and random forest classifiers, and employ fivefold cross-validation for training. The predictive power of the models will be assessed by calculating the area under the receiver operating characteristics curve. Statistical analyses will be conducted using R V.3.6.2. A two-tailed value of p<0.05 will be considered statistically significant.
Patient and public involvement
Patients and the public are not involved in the design, recruitment or conduct of this study. While we do not plan to inform every individual participant of the study results, we will disseminate the findings to the public through community outreach and public engagement initiatives.
Ethics and dissemination
This study was approved by the Biomedical Research Ethics Committee of West China Hospital of Sichuan University (No. 20232422). All research organisations contributing data for the iWAVE Study will comply with this ethical approval, and any modifications to the study protocol will be submitted for further ethical review. All participants will be required to provide written informed consent, and patient data will be stored in deidentified form at the Information Center of West China Hospital of Sichuan University. All protocols will be conducted in compliance with the Declaration of Helsinki. Access to the study data will be restricted to researchers and ethics committees associated with the study.
The results will be disseminated in various formats, including conference abstracts, posters, presentations and peer-reviewed scientific journals. All of the results generated in this study will be cohort data, ensuring that participants cannot be individually identified. Additionally, the findings will be reported to local governments and health and wellness committees to inform policy development, as well as to medical research centres and units that participate in and support the cohort study.
Discussion
To the best of our knowledge, this is the first cohort study conducted across multiple medical consortiums led by a regional centre hospital in Western China. It focuses on the diagnosis, treatment and prognostic outcomes in a population with VHD in the region, providing comprehensive data on risk factors, diagnostic and therapeutic management, physical status, and whole-life cycle follow-up experiences.
Although national databases for cardiac surgery have long been established internationally, there are notable differences in population and disease characteristics, as well as healthcare public service systems, among China, Europe and the USA.14 26 Therefore, the iWAVE cohort database accounts for the unique population characteristics of western China and focuses on screening variables related to the diagnosis, treatment and prognosis of VHD.20 The iWAVE database will enable continuous monitoring of the entire lifespan of patients with VHD within this population. A series of analysis reports based on the iWAVE cohort study will provide a comprehensive overview of the incidence, demographics, care pathways and whole-life cycle follow-up of VHD in the population of western China. This report will identify risk-modifying factors that influence outcomes, establish benchmarks among cardiac centres, and propose system-wide strategies aimed at improving the survival of patients with VHD. Additionally, the database is promising for assisting both the public and the government in prioritising primary prevention and lifelong follow-up of patients with VHD. Successful outcomes may motivate other cardiovascular medical centres across the country to participate, potentially establishing a national platform for monitoring cardiovascular surgical care in western China, facilitating research projects, establishing benchmarking standards and identifying areas for potential quality improvement.
This study has several limitations. First, the medical centres involved are confined to western China, resulting in a cohort that represents only the VHD population in that region and fails to capture the broader characteristics of VHD across China. However, as the study progresses, we aim to broaden the inclusion criteria to encompass more large medical centres in non-western China, thereby diversifying the geographical distribution of the study population over time. Second, the current biomarkers lack specificity in predicting VHD disease progression, and with the ongoing advancement of transcatheter valve interventions, obtaining patient biospecimens has become increasingly challenging. Therefore, we plan to collect peripheral venous blood samples and urine and stool samples from a community-based natural population cohort, as well as samples of valvular and/or myocardial tissue from patients who undergo median open-heart surgery at the medical centres involved in this study, to explore the microscopic mechanisms of VHD disease. Third, the design of the cohort study limits our ability to implement various medical interventions for conducting long-term prognostic correlation analyses. Nonetheless, incorporating a multicentre cohort population could provide a sufficiently large sample size for propensity score-matched analyses aimed at exploring the relationships between target variables and outcome events. Finally, significant time and resources were dedicated during the prestudy phase to ensure the accuracy and completeness of the data. In future sustainability studies, the iWAVE cohort study is committed to streamlining the data reporting process and automating data collection.
Data availability statement
We invite national and international collaborations for projects concerning various aspects, including but not limited to disease screening strategies, diagnostic and therapeutic decisions, and long-term functional trajectories. Researchers are encouraged to apply for data access by contacting the chief investigator (YG) at drguoyq@wchscu.cn, providing details of the study objectives and proposals.
Ethics statements
Patient consent for publication
Ethics approval
The study protocol was approved by the Biomedical Research Ethics Committee of West China Hospital of Sichuan University (No. 20232422). All participants will be required to provide written informed consent. The study findings will be disseminated via publications in peer-reviewed journals and presentations at scientific conferences.
Acknowledgments
The authors thank all hospitals, principal investigators, healthcare professionals, researchers and patients involved in the iWAVE cohort study for their contributions. The authors also thank the Integrated Care Management Centre of West China Hospital of Sichuan University for their support in providing a whole life cycle management system. The authors also thank the Information Centre of Sichuan University for their contributions in supplying medical electronic case records, developing the EDC system and enhancing data analysis and quality control.
References
Footnotes
XL and YW contributed equally.
Contributors All authors contributed to the conception or design of the work. YW, XL and TC contributed to the acquisition, analysis or interpretation of data for the work. SJ contributed to analysis for the work. LL contributed to the interpretation of data for the work. YW and XL drafted the manuscript. YZ and YG critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy. YW is the guarantor.
Funding This work was supported by National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (No. Z2024YY001).
Map disclaimer The inclusion of any map (including the depiction of any boundaries therein), or of any geographical or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.