Article Text
Abstract
Introduction Non-communicable diseases (NCDs) present a significant health challenge globally, especially in low- and middle-income countries (LMICs). Effective Implementation Research (IR) is vital in addressing this challenge, with stakeholder engagement playing a crucial role. However, the landscape of stakeholder engagement in NCD IR within LMICs faces unique challenges, including resource constraints and power imbalances. Despite these challenges, stakeholder engagement offers substantial benefits, including improved research relevance and sustainability of interventions.
Methods and analysis This scoping review aims to comprehensively describe the current practices of stakeholder engagement in NCD IR within LMICs. Employing a two-stage screening process and a thematic synthesis approach based on the International Association for Public Participation Spectrum of Public Participation, the review will analyse studies meeting predefined eligibility criteria. A rigorous search strategy will be implemented across identified electronic databases and grey literature sources, including published studies from 2011 to present. Data will be charted using a standardised form, and information regarding study characteristics, NCD focus, LMIC context, stakeholder engagement method and reported outcomes/findings will be collected. This scoping review will follow a standard protocol adhering to the methodological framework outlined by Arksey and O'Malley to comprehensively map existing evidence on stakeholder engagement in NCD IR within LMICs.
Ethics and dissemination Ethical considerations involve respecting original authors, maintaining integrity and transparency, managing data ethically and disclosing conflicts of interest. Dissemination will occur through publication in peer-reviewed journals, conference presentations, open-access repositories, policy briefs, stakeholder engagement activities and social media platforms.
Registration This scoping review protocol is registered on Open Science Framework, with the Digital Object Identifier 10.17605/OSF.IO/ACQ52, ensuring transparency and accountability in the research process.
- Implementation Science
- Review
- Chronic Disease
- Patient Participation
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
Rigorous two-stage screening process with independent reviewers.
Comprehensive search strategy, including electronic databases and grey literature.
Clear eligibility criteria and standardised data charting form.
Potential for missing relevant studies due to database coverage limitations and language restrictions.
Reliance on reported information in included studies.
Introduction
Non-communicable diseases (NCDs) are chronic conditions that are a result of a combination of genetic, physiological, environmental and behavioural factors.1 Globally, they are responsible for 71% of all deaths, with cardiovascular diseases, diabetes, cancers and chronic respiratory diseases (such as chronic obstructive pulmonary disease and asthma) making up over 80% of premature deaths caused by NCDs.1 These diseases contribute substantially to morbidity, mortality and healthcare burdens in low- and middle-income country (LMIC) settings.2 To effectively address this challenge, robust implementation research (IR) is crucial.
A key aspect of successful IR is stakeholder engagement.3 Effective stakeholder engagement fosters collaboration, improves research relevance and acceptability and ultimately enhances the implementation success of health interventions.4
For the purposes of this scoping, we will adopt the definitions by Concannon et al.5 A ‘stakeholder’ is defined as an individual or group who is responsible for or affected by health- and healthcare-related decisions.5 The term ‘stakeholder engagement’ will be used to describe activities the researcher can undertake in this relationship, and ‘stakeholder involvement’ refers to the activities that either researchers or stakeholders can engage in.5 The term ‘roles’ will be used to describe the research activities in which stakeholders may participate, while ‘modes of engagement’ will refer to the processes by which researchers and stakeholders interact with each other.5 It is important to note that studying the stakeholders’ engagement is different from studying their views.
IR is the scientific investigation of how to carry intentions into effect, such as health policies, programmes or practices.6 It seeks to understand how, why and under what conditions interventions work in real-world settings, making it distinct from other forms of research that might aim to control or eliminate external influences.6 7
A key feature of IR is its emphasis on context—social, cultural, political, economic and institutional factors—acknowledging that these elements significantly influence the success or failure of an intervention.6 IR involves not only the production of knowledge but also the active engagement of stakeholders, such as policymakers, health practitioners and communities.7 These stakeholders are not just end-users but often participate in the design and implementation phases, ensuring that interventions are tailored to local needs and conditions.6
IR assesses several key outcome variables to measure the success of implementation, including acceptability, feasibility, fidelity, sustainability and cost.6 It also considers ‘implementation strategies’—methods designed to enhance the adoption of health interventions.6
Moreover, IR recognises the complex adaptive systems in which health interventions are implemented.6 7 As such, it often requires flexible research methods capable of capturing the iterative, evolving nature of implementation processes.6 The use of qualitative, quantitative and mixed methods is common in IR to provide a comprehensive understanding of the factors influencing implementation, along with potential unintended consequences.6
In LMICs, stakeholder engagement for NCDs IR is confronted with distinctive challenges. Resource constraints within LMIC healthcare systems pose a significant obstacle, often hindering the allocation of personnel and funding for stakeholder engagement activities.8 9 Additionally, weak health infrastructure and limited research capacity in some LMICs can impede the identification and effective engagement of relevant stakeholders. Engaging stakeholders throughout the research process helps ensure that interventions are designed with their needs, priorities and contexts in mind, thereby leading to more relevant and adaptable solutions.10 Actively involving stakeholders in research design, implementation and evaluation fosters a sense of ownership, contributing to the long-term sustainability of NCD interventions.5 10 Moreover, stakeholder engagement allows for the identification of contextual factors that might influence intervention effectiveness and facilitates the adaptation of strategies to different LMIC settings.3 Collaboration with local stakeholders also strengthens research capacity within LMICs by fostering knowledge exchange and skills development.3 10
Rationale for the scoping review
The rationale for conducting the scoping review is driven by the recognised importance of stakeholder involvement in NCDs IR. Despite this recognition, a comprehensive understanding of how stakeholders are presently engaged throughout the research process in LMICs is lacking. Currently, there exists an absence of a clear picture regarding the frequency, phases, types of stakeholders and methods of engagement within the NCD IR landscape.
The field of stakeholder engagement in NCD IR within LMICs is likely diverse and heterogeneous. Using a scoping review methodology allows for broad exploration and mapping of the existing literature.11–14
Our primary aim in undertaking this scoping review is not to assess the effectiveness of specific interventions but rather to comprehensively describe current practices. Scoping reviews are particularly adept at summarising and disseminating existing knowledge.11 12
Through conducting this scoping review, we anticipate gaining valuable insights into the current state of stakeholder engagement in NCD IR within LMICs. This knowledge will serve to inform the development of more effective and standardised approaches to stakeholder involvement, ultimately contributing to more impactful NCD research and interventions.
Methods and analysis
Patient and public involvement statement
None.
Protocol and registration
This protocol is registered on Open Science Framework (OSF), with the Digital Object Identifier (DOI) 10.17605/OSF.IO/ACQ52, ensuring transparency and accountability in the research process.
Study period
The scoping review is scheduled to begin in October 2024, once the protocol is approved for publication in a peer-reviewed journal, and is expected to be completed by January 2025. These dates encompass the entire review process, including literature search, screening, data extraction, analysis and dissemination. The most recent search for eligible studies will be conducted on 21 December 2024.
Eligibility criteria
To ensure a focused and relevant review, we will employ the following inclusion and exclusion criteria for selecting studies to be included in this scoping review.
Inclusion criteria
Study design: we will include studies that employ any qualitative, quantitative or mixed-methods research design.
Population: the study population must focus on NCDs. For the purpose of this scoping review, we will mainly focus on the four major contributing NCDs: cardiovascular diseases, diabetes, cancers and chronic respiratory diseases.1
Context: the research must be conducted in LMIC settings as defined by the World Bank.15
Intervention/Focus: the study must explicitly address NCD IR.
Publication status: we will include both peer-reviewed and grey literature sources (eg, dissertations, theses and reports from governmental or non-governmental organizations) to capture a comprehensive understanding of stakeholder engagement practices.
Language: we will restrict the search studies published in English to ensure feasibility and maintain consistency within the review team. However, we acknowledge the potential for language bias and explore potential mitigation strategies during the data analysis phase.
Time frame: 2011–2024. We have chosen 2011 as the starting year for this scoping review, recognising it as a pivotal moment in global NCD prevention and control efforts.16 In 2011, the WHO published a Prioritised Research Agenda for the Prevention and Control of NCDs, 17 which highlighted the critical areas of research. That same year, the United Nations High-Level Meeting on NCD Prevention and Control underscored the existence of cost-effective NCD interventions and opportunities for global action, culminating in the endorsement of the Global Action Plan for the Prevention and Control of NCDs 2013–202018 by the 66th World Health Assembly.
Exclusion criteria
Study design: protocols and reviews will be excluded.
Information sources
To ensure a comprehensive search and capture relevant studies on stakeholder engagement in NCD IR within LMICs, we will use the following information sources: EMBASE, PubMed (Ovid), MEDLINE (EBSCOhost), CINAHL (EBSCOhost), Web of Science (WOS) and Global Index Medicus, that includes Regional Indexes AIM (AFRO), LILACS (AMRO/PAHO), IMEMR (EMRO), IMSEAR (SEARO) and WPRIM (WPRO).
Search strategies
We will develop a comprehensive search strategy using a combination of controlled vocabulary (Medical Subject Headings - MeSH - terms for PubMed/MEDLINE and subject headings for other databases) and keyword terms. The search strategy will be tailored to each specific database and will incorporate the following key concepts.
NCDs (eg, cardiovascular diseases, diabetes, chronic respiratory diseases and cancers).
IR
LMICs.
In recognising the significance of the planning stage in IR, we will specifically identify projects employing cocreation and codesign methodologies. Our approach to identifying relevant studies will involve using targeted keywords associated with cocreation and codesign, alongside IR, to gather a comprehensive range of literature, and analysing the reference lists of articles focused on IR to identify earlier studies that have used cocreation or codesign approaches in their planning phases (online supplemental file 1).
Supplemental material
Additional sources
We will also search the grey literature to identify relevant studies not indexed in traditional academic databases. This may involve searching the websites of relevant organisations (eg, WHO and World Bank), LMIC government health department websites and repositories for theses and dissertations.
We will perform handsearching of reference lists from included studies to identify potentially relevant studies not captured through the electronic database searches.
Selection of sources of evidence
To ensure a systematic and unbiased approach to selecting relevant studies for this scoping review, we will implement a two-stage screening process.
Stage 1: title and abstract screening
All retrieved citations from electronic database searches and other sources, including grey literature and reference list handsearching, will be uploaded into a reference manage software, such as EndNote, Mendeley or Zotero. Duplicates will be removed during this process. Following this, two independent reviewers will screen the titles and abstracts of all retrieved citations according to the predefined eligibility criteria. Any discrepancies between the reviewers will be resolved through discussion or by consulting a third reviewer to achieve consensus.
Stage 2: full-text screening
The full text of all studies deemed potentially relevant after the title and abstract screening will be retrieved. Two independent reviewers will then assess the full text of each article against the eligibility criteria, conducting a more in-depth analysis of the study design, population, intervention or focus, context and publication status. Reviewers will use a standardised data extraction form to ensure a consistent evaluation of the eligibility criteria. Any disagreements arising during the full-text screening will be resolved through discussion or by involving a third reviewer for a final judgement.
Data charting process
This scoping review adheres to the methodological framework outlined by Arksey and O'Malley11 to comprehensively map existing evidence on stakeholder engagement in NCD IR within LMICs. Here is how we will apply the framework throughout the review process.
Stage 1—identifying the research question: ‘What are the current practices and experiences of stakeholder engagement in NCD IR within LMICs?’
Stage 2—identifying relevant studies: we will use a comprehensive search strategy using electronic databases and will search for grey literature through relevant organisation websites and reference list handsearching. We defined clear inclusion and exclusion criteria to ensure that the retrieved studies align with the research question.
Stage 3—study selection: a two-stage screening process will be implemented, as described in the ‘selection of sources of evidence’ section above.
Stage 4—charting the data: a standardised data charting form will be developed to capture key information from the included studies.
Stage 5—collating, summarising and reporting the results: we will narratively synthesise the findings, providing an overview of the current landscape of stakeholder engagement in NCD IR within LMICs. The scoping review findings will be reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews guidelines to ensure transparency and completeness.
Stage 6—consultation: while not a formal stage in Arksey and O'Malley’s framework, we have decided not to engage with stakeholders in this phase of the review. Instead, we will rely on the existing literature and frameworks to guide our analysis and conclusions.
Data items
To comprehensively capture information on stakeholder engagement practices in NCD IR within LMICs, we will extract data from the included studies using a standardised data charting form. Here is an outline of the key data items we will be seeking.
Study characteristics
Author(s) and publication year.
Study title.
Study design (eg, qualitative, quantitative and mixed methods).
NCD focus
Specific NCDs addressed in the research (eg, cardiovascular disease and diabetes).
LMIC context
Region or specific LMIC country where the research was conducted.
Stakeholder engagement methods
Types of stakeholders involved (eg, policymakers, healthcare providers, community members and researchers).
Specific methods used for stakeholder engagement (eg, community advisory boards, focus group discussions and workshops).
Phases of the research process where stakeholder engagement occurred (eg, planning, implementation and evaluation).
Specific role of the stakeholder involved.
Reported outcomes/findings
Impact of stakeholder engagement on the research process or intervention outcomes (if evaluated).
Any other relevant findings related to stakeholder engagement.
Synthesis of results
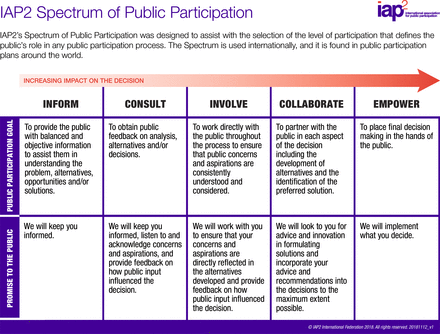
Following data extraction from the included studies using the standardised data charting form, we will employ a thematic synthesis approach to analyse and synthesise the findings. We will use the International Association for Public Participation (IAP2) Spectrum of Public Participation as the primary framework to analyse the level and quality of stakeholder engagement in the selected studies. The IAP2 Spectrum provides a well-established model for categorising public participation across five levels: inform, consult, involve, collaborate and empower (figure 1). These levels represent a continuum of engagement, from minimal stakeholder involvement (inform) to full decision-making power (empower).
IAP2 for public participation framework. IAP2, International Association for Public Participation.
The spectrum is defined as follows.
Inform: stakeholders are provided with information about the intervention but have no direct involvement in decision-making.
Consult: stakeholders are asked for feedback on specific aspects of the intervention.
Involve: stakeholders are actively involved in the decision-making process, but the ultimate decisions rest with the project implementers.
Collaborate: stakeholders work in partnership with the implementers, sharing responsibility for decision-making and outcomes.
Empower: stakeholders have control over decision-making and the design and implementation of the intervention.
The IAP2 Spectrum will be used to categorise the type and extent of stakeholder engagement reported in each study reviewed. During the data extraction phase, we will assess the level of engagement by coding each study according to where it falls on the IAP2 Spectrum. This structured coding will allow us to assess the frequency and nature of each level of engagement across studies.
Using the identified themes, we will develop a narrative synthesis that provides a comprehensive overview of stakeholder engagement practices in NCD IR across LMIC settings. This synthesis will integrate findings from the studies, highlighting the diversity of approaches to stakeholder engagement, the reported benefits and challenges of these approaches at different levels of the IAP2 Spectrum and the impact of engagement levels on research processes, outcomes and sustainability of NCD interventions.
We will also present the findings visually through tables or charts to summarise key themes and patterns. For example, we will use tables to display the distribution of studies across the IAP2 Spectrum, showing how frequently different levels of stakeholder engagement were employed. Furthermore, visual representations may be used to highlight any associations between engagement levels and specific outcomes, such as intervention success or stakeholder satisfaction.
Additionally, we will incorporate direct quotes from the included studies to illustrate key points and provide a richer understanding of stakeholder experiences. These quotes will help bring context to the themes and offer insights into how stakeholders perceived their level of involvement in the research processes.
By synthesising the data through the lens of the IAP2 Spectrum, this review will provide a deeper understanding of how different stakeholder engagement practices affect NCD IR in LMICs and offer valuable insights for future research and practice.
Potential findings and implications
By highlighting the current practices of stakeholder engagement in NCD IR, the findings could provide insights into how to better integrate stakeholders—such as patients, healthcare providers and policymakers—into the research process. This might lead to more culturally appropriate and context-specific interventions, increasing their relevance and effectiveness in LMICs. The review could identify successful strategies for involving stakeholders in policy development, which may help guide future policy frameworks for NCD management in LMICs. The review may uncover gaps in the extent or quality of stakeholder engagement across different stages of IR. The findings could highlight how stakeholder engagement practices address or fail to address equity and inclusiveness. This is particularly important in LMICs, where vulnerable populations often face systemic barriers to participation in health research. The results of this scoping review could serve as a roadmap for future NCD IR by identifying best practices, challenges and key factors influencing successful stakeholder engagement. Researchers may use this knowledge to design studies that are more effective in LMIC settings, contributing to global knowledge on NCD prevention and control.
Limitations
This scoping review has inherent limitations that need to be considered when interpreting the findings. The search strategy may not capture all relevant studies due to database coverage, language restrictions and keywords choices. Human error and potential bias could arise from relying on two independent reviewers, despite measures to ensure objectivity. Our assessment is limited to explicitly reported information, which may not fully capture the depth of stakeholder engagement activities. Thematic synthesis involves subjective interpretation, and reporting bias may skew the focus towards positive outcomes, as studies with favourable results are more likely to be published.
Ethics and dissemination
This scoping review will involve the collection and analysis of data from previously published studies. As such, it does not require direct interaction with human participants or access to personal, confidential data. Consequently, ethical approval is not necessary for the conduct of this review. However, we will adhere to the highest standards of ethical research practices by ensuring respect for original authors by acknowledging and citing all sources and original authors whose work is included in the review, integrity and transparency by reporting our methods and findings to allow reproducibility and verification by other researchers, using reference management software to organise and handle the data systematically to ensure data integrity and confidentiality and by disclosing any potential conflicts of interests among the review members maintaining objectivity and impartiality in the review process.
The findings of this scoping review will be disseminated through multiple channels to reach a broad audience. We will submit the final review manuscript to a high-impact, peer-reviewed journal in the field of public health, implementation science or global health. The research protocol and final report will be made available on the OSF to promote transparency and accessibility. A DOI will be obtained and published to ensure permanent and citable access to the work. We will develop policy briefs summarising the key findings and implications for stakeholder engagement in NCD IR within LMICs. These briefs will be targeted at policymakers and practitioners to inform evidence-based decision-making and practice. To reach a wider audience, we will use social media platforms (eg, Twitter and LinkedIn) and contribute to relevant blogs and online forums discussing NCDs and IR.
Ethics statements
Patient consent for publication
Footnotes
Contributors MJCP conceptualised the research protocol and is responsible for coordinating all phases of the research. He oversees the analysis and takes the lead on writing the final report and is the guarantor for the overall content of the work. AC and PE contributed to refining the research protocol. They are responsible for data collection and will assist in the data analysis and refining the final report. In accordance with the journal’s policy on AI usage, we would like to transparently disclose the use of OpenAI’s GPT-4, a large language model, during the preparation of this manuscript. AI technology was employed to assist in drafting and refining certain sections of the manuscript, particularly to enhance the clarity of complex explanations, ensure consistency in language and style, and generate initial drafts of non-technical content. The goal of using AI was to improve the overall readability and coherence of the manuscript. During the drafting process, GPT-4 was used to generate initial versions of specific sections, such as the introduction, which helped structure ideas and present them in a clear and concise manner. It also assisted in reviewing the manuscript to ensure that the language and style remained consistent throughout. Additionally, GPT-4 was used to help draft responses to reviewer comments by suggesting ways to address specific points and ensuring the responses were well-organised. The input provided to the AI consisted of specific prompts related to content that required drafting or revision, along with detailed instructions for the expected output. The AI-generated content was treated as a draft or suggestion and was subsequently reviewed, refined and validated by the authors. The final manuscript fully reflects the authors’ original research and ideas, with the AI used strictly as a tool to support the writing process.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.