Article Text
Abstract
Introduction Infants born very preterm (VPT, <32 weeks’ gestation) are at increased risk for neurodevelopmental impairments including motor, cognitive and behavioural delay. Parents of infants born VPT also have poorer mental health outcomes compared with parents of infants born at term.
We have developed an intervention programme called TEDI-Prem (Telehealth for Early Developmental Intervention in babies born very preterm) based on previous research. TEDI-Prem aims to improve neurodevelopmental outcomes and parental well-being in children born VPT. Here we present the protocol outlining a multicentre, pragmatic, parallel-group, randomised controlled trial to determine the efficacy of TEDI-Prem plus usual care, compared with usual care alone.
Methods and analysis We will recruit 466 VPT infants from the neonatal units of five hospitals in Victoria, Australia. Participants will be randomised, stratified by site of recruitment and multiple births, to TEDI-Prem plus usual care or usual care alone. The TEDI-Prem intervention programme involves 13 sessions across three phases. Phase 1 commences in the neonatal unit with four face-to-face sessions with parent/s and a physiotherapist/occupational therapist. Once discharged from the hospital, sessions across phases 2 and 3 (six and three sessions, respectively) continue via telehealth until infants are 12 months’ corrected age (CA).
The primary outcome is the Bayley Scales of Infant and Toddler Development-fourth edition (Bayley-4) Motor Composite Score at 12 months’ CA. Secondary outcomes address other neurodevelopmental domains (Bayley-4 cognitive and language composite score; Infant Toddler Social Emotional Assessment), parental mental health (Depression Anxiety and Stress Scale 21), parent–child interaction (Emotional Availability Scale) and programme cost-effectiveness which encompasses parent quality of life (Short-Form Six-Dimension Quality of Life) and child quality of life (EuroQol Toddler and Infant Populations measure) at 12 and 24 months’ CA.
Mean differences between groups will be examined using linear regression for continuous outcomes and logistic regression for binary outcomes. All models will be fitted via generalised estimating equations to account for multiple births and adjusted for the hospital sites.
Ethics and dissemination This trial has Royal Children’s Hospital Human Research and Ethics Committee approval (HREC/67604/RCHM-2020) with specific site approval for all participating sites. Findings will be disseminated through peer-reviewed publications, conference presentations, digital and print media and to participants.
Trial egistration number This trial is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12621000364875).
- Telemedicine
- Clinical Trial
- Neonatal intensive & critical care
- Developmental neurology & neurodisability
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Telemedicine
- Clinical Trial
- Neonatal intensive & critical care
- Developmental neurology & neurodisability
STRENGTHS AND LIMITATIONS OF THIS STUDY
We have adapted and combined two pilot-tested intervention programmes targeted at very preterm infants and their families.
The intervention sessions are delivered in the hospital in phase 1 and post-discharge in phases 2 and 3 with face-to-face and telehealth modes used, respectively. This increases access to intervention for families, including those who face barriers to service use such as living in regional or rural communities.
This study will recruit a large sample size from five out of six Victorian neonatal intensive care units. The intervention is conducted in a high-income setting and therefore may not be generalisable to low-middle-income settings.
Introduction
Worldwide, over 2.5 million babies are born very preterm (VPT, <32 weeks’ gestation) each year.1 In Australia alone, there are approximately 5000 VPT births each year, and while most of these infants survive,2 over 50% of VPT infants will have a neurodevelopmental impairment.3 4 The neurodevelopmental deficits resulting from early birth may compromise physical function, academic achievement and quality of life.5–7 While survival rates are improving in children born VPT, the rates of neurodevelopmental impairments are not improving over time8 and are associated with substantial economic costs.9 Further, following the VPT birth, parents have higher rates of mental health problems than their peers with term-born infants, which is itself associated with poorer child outcomes.10 11
VPT birth occurs during a critical period of central nervous system development, with the first 1000 days of life the most dynamic and rapid period of brain development, setting the foundation for all future neural development.12 13 This period of brain development is particularly vulnerable to adverse environmental events and exposures, including preterm birth. The VPT infant is often critically ill and requires invasive interventions in the neonatal intensive care unit (NICU). Together with their early illness, this care environment, while necessary to support life, can disrupt brain maturation. Further, the NICU environment can alter the parent–infant relationship, infant sensorimotor experiences and offers limited opportunities for social, motor, cognitive and language interactions.14–16 Parents are more likely to experience higher anxiety and depression after preterm birth than peers who have babies born at term,10 17 18 which contributes to an altered parent–child relationship and increases the risk for poorer developmental outcomes for VPT children.5 19 VPT birth alone carries heavy healthcare costs in the newborn period, but longer-term costs are much higher when children have developmental or health problems.20 Averting these long-term sequelae for VPT infants through early intervention has potential economic benefits in health and education.9 While medical advances continue to improve neonatal care,21 clinical rehabilitation for these high-risk infants has not kept pace with advances in basic science and developmental theory to improve outcomes for infants and their parents.22–24
In a Cochrane review of early developmental interventions post hospital discharge, our team demonstrated that early intervention had a moderate effect on cognitive development (standardised mean difference (SMD)=0.32, 95% CI 0.16, 0.47) but a smaller effect on motor development (SMD=0.10, 95% CI 0.01, 0.19) up to 3 years of age compared with usual care.25 A recent systematic review of interventions commencing in the NICU found that parent-delivered motor interventions were more effective than other early developmental interventions in improving motor and cognitive outcomes in the short-term.26 Neurorehabilitation and neuroplasticity research supports high repetitions of task-specific activities to enhance learning and establish neural pathways in infants.24 27 It has also been shown that intense interventions that involve parents are more effective at promoting neurodevelopmental outcomes than low-intensity interventions that focus only on the therapist and infant.25 28 Given that VPT infants are at high risk of motor, cognitive and behavioural impairments, there is an even greater need for high repetitions and intensity to enhance learning in infants born VPT, and thus interventions must engage parents to achieve a high dose.25
The provision of high-intensity intervention during a time of maximum neuroplasticity, as supported by the basic science and theoretical literature,12 29 is not currently available to VPT infants in Australia and in many countries internationally. Our research has shown that the majority of preterm infants do not receive timely early intervention, and those with higher family social risk (socioeconomic disadvantage) are less likely to receive intervention.30 Further barriers to accessing effective early intervention for preterm infants and their families include a ‘wait and see’ approach rather than a preventative model, lack of access to appropriately trained health professionals (particularly for rural, remote and/or socioeconomic disadvantaged families), provision of generic interventions rather than targeting the needs of the individual infant and family, limited funding for services and lack of communications between families and health professionals.30 31 Consequently, many preterm infants do not receive intervention during a critical developmental window for promoting functional neural pathways and improving future function.32
Two promising interventions for preterm infants and their families that have been recently trialled by our team include ‘SPEEDI’ and ‘e-prem’. SPEEDI (Supporting Play Exploration and Early Development Intervention) is a face-to-face early intervention programme, developed by author SD and colleagues in Virginia, USA, and has been trialled in two pilot randomised controlled trials (RCTs) (one in the USA33 34 and one in Australia).35 Although both SPEEDI pilot studies were underpowered for determining efficacy, there was a trend for infants in the SPEEDI group to have better motor, cognitive and language scores on the Bayley-3 at four (Australian pilot) and six (USA pilot) months’ corrected age compared with those in the control group. Both studies determined the SPEEDI intervention was feasible to deliver. A protocol paper for a larger trial evaluating the efficacy of SPEEDI was published in 2020.36
e-prem was developed by authors KT, PJA and AJS based on their research on early intervention for preterm infants and is an adaptation of the VIBeS Plus programme.29 e-prem involves initial face-to-face intervention followed by a web-based intervention, with age-based online modules completed over the first year of life, supported by clinicians via telephone. In a pilot RCT of e-prem compared with usual care, 100 preterm infants were followed-up at 24 months’ corrected age. Parent–infant interaction was assessed using the Emotional Availability Scale at 24 months’ corrected age (CA). Parents in the intervention group scored higher than those in the control group (reflecting more optimal outcomes) on maternal structuring (mean difference=0.72, 95% CI=0.21, 1.22), and children in the intervention group scored higher than those in the control group on child responsiveness (coefficient=0.58, 95% CI=0.03, 1.13), and child involvement (coefficient=0.62, 95% CI=0.09, 1.15).37
With advances in digital technologies and improved accessibility, telehealth (defined as the use of information and communication technologies to provide clinical services from a distance) has made it possible for therapeutic interventions to be delivered directly to families in their homes.38 Telehealth has the added benefit of reducing the risk of exposure to other illnesses and infections; especially for patients who may have compromised or weakened immune systems such as those born VPT.
The results of our previous research demonstrated that while using telehealth, e-prem alone did not appear to improve motor or cognitive outcomes in VPT infants but showed an improvement in parent mental health and the parent–child relationship. SPEEDI showed preliminary evidence of effectiveness in improving neurodevelopmental outcomes but has not been delivered via telehealth and does not target parent mental health and well-being. In order to give the intervention the best chance of demonstrating effectiveness, we have consolidated the effective components from both previous interventions and developed an innovative approach to early intervention to enhance neurodevelopment called TEDI-Prem (Telehealth for Early Developmental Intervention in babies born very preterm). We now plan to test the efficacy of the TEDI-Prem intervention in a randomised trial.
Aims
The primary aim of this RCT is to determine the efficacy of TEDI-Prem plus usual care compared with usual care alone to improve motor outcomes at 12 months’ CA in children born VPT. Secondary aims are outlined in box 1.
Secondary aims
To determine the efficacy of Telehealth for Early Developmental Intervention in babies born very preterm plus usual care compared with usual care alone to improve:
Motor outcomes at 24 months’ corrected age (CA) for infants born very preterm (VPT).
Cognitive, language, behaviour and quality of life at 12 and 24 months’ CA for infants born VPT.
Parent well-being (anxiety and depression), quality of life and self-efficacy at 12 and 24 months’ CA for parents of infants born VPT.
Parent–infant interaction at 12 and 24 months’ CA between infants born VPT and their primary caregiver.
Healthcare utilisation at 12 and 24 months’ CA for infants born VPT.
Cost-effectiveness over 24 months.
Methods and analysis
Study design
A multicentre, pragmatic, parallel-group, RCT designed according to SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guidelines,39 the principles of Good Clinical Practices, the Template for Intervention Description and Replication checklist and guide (table 1),40 and in adherence to the National Statement and Australian Code for Responsible Research Conduct.
Template for Intervention Description and Replication checklist: how TEDI-Prem differs from usual care
Study setting
Trial recruitment will be undertaken in the NICU or special care nursery (SCN) of five hospitals in the state of Victoria, Australia, including The Royal Women’s, The Royal Children’s, Monash Children’s, Joan Kirner Women’s and Children’s and the Northern Hospitals. These centres are chosen as they encompass five of the six main sites of care for VPT infants in Victoria.
Participants
VPT infants and their parent/s (at least one parent must participate in the trial) will be recruited from Victoria, Australia. Throughout this protocol, the term ‘parent’ will be used to refer to any individual who provides primary care to the infant (eg, grandparent, foster carer). Inclusion and exclusion criteria are outlined in box 2.
Eligibility for inclusion in the randomised controlled trial
Inclusion criteria
Infant born <32 weeks’ gestation.
Infant medically stable and not ventilator-dependent at recruitment (minimum age for enrolment 32 weeks’ and maximum 40 weeks’ postmenstrual age).
Have one parent who speaks and can read English (as the video content and intervention materials are in English).
Able to participate in an early intervention programme for a 12-month period.
Able to attend primary outcome assessment at 12 months’ corrected age.
Exclusion criteria
Non-English speaking primary parent.
Infant with a diagnosis of a congenital abnormality known to affect neurodevelopment, who require specific intervention, such as infants with trisomy 21.
Families who are planning to move overseas/interstate prior to the primary outcome assessment at 12 months’ CA.
Parents not wanting to engage in telehealth intervention.
Sample size calculation
The primary outcome is the Bayley-441 Motor Composite Score at 12 months’ CA, which has a mean of 100 and an SD of 15. While there is no reported minimum clinically important improvement on the Bayley-4, a difference of five points represents an SMD of 1/3 between groups and is considered clinically important and consistent with previous RCTs in the field that have changed clinical practice.4 In order to achieve 90% power to identify an SMD of 1/3 based on a two-sided t-test with a 5% significance level, we require 190 participants per group assuming that the observations are independent. Given that our sample will be children born <32 weeks we would expect approximately 20% to be multiple births. Assuming an intraclass correlation coefficient of 0.2 between multiple births, this equates to a design effect of 1.04, hence we need to recruit 198 per group to have 190 effective participants. Allowing for a 15% loss to follow-up (conservative estimate—previous studies by our team have >90% follow-up), we aim to randomise a total of 466 infants over 32 months. In an Australian pilot study of SPEEDI, >85% of eligible infants were recruited over a 3-month period at a single site (n=17 infants).35 Over 700 infants are born VPT in Victoria each year and we are recruiting from four out of five Victorian NICUs, along with a large special care nursery, making our sample size target very achievable. Further, the fact that the intervention involves telerehabilitation will allow participants to be recruited without geographical limitations.
Recruitment
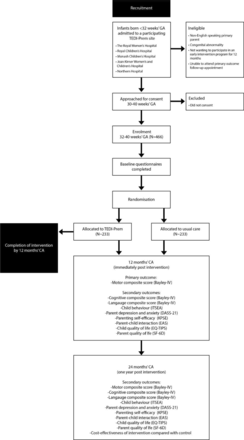
Eligible infants will be identified by a member of the research team at the participating sites while infants are in the NICU or SCN. With approval from the infant’s clinical team, when the infant is considered medically stable, is not ventilator dependent (can be on continuous positive airway pressure) and is between 30 and 40 weeks’ gestation, parents of eligible infants will be approached by a member of the research team and provided with the Participant Information and Consent Form for the trial (see online supplemental material 1). In the event that COVID-19 precautions prohibit the research team from entering the NICU or SCN at a participating site, a clinical team member will approach families to introduce the study and gain verbal consent for a research team member to contact them to explain the study in more detail. The research team member will give a verbal explanation of the trial, including a description of the trial processes, the voluntary nature of the trial and that a decision to participate, or not, will not affect the standard clinical care they and their infant receive. See figure 1 for an overview of participant recruitment and follow-up.
Supplemental material
Participant flow chart. GA, Gestational age; Bayley-4, Bayley Scales of Infant and Toddler Development-fourth edition; CA, corrected age; DASS-21, Depression Anxiety and Stress Scale 21; EAS, Emotional Availability Scale; EQ-TIPS, EuroQol Toddler and Infant Populations; ITSEA, Infant Toddler Social Emotional Assessment; KPSE, Karitane Parenting Confidence Scale; SF-6D, Short-Form Six-Dimension Quality of Life; TEDI-Prem, Telehealth for Early Developmental Intervention in babies born very preterm.
Data collection, management and access
Data for this trial will be collected and entered using electronic data collection forms which will be completed by the parent and researchers and entered via The University of Melbourne’s Research Electronic Data Capture42 43 database system. All data will be de-identified, with all participants allocated a unique trial identification number. Intervention sessions will be recorded using Zoom Video Communications (Zoom). All files containing private or confidential data will be stored only in locations accessible to designated members of the research team on secure networks that are backed up nightly.
Baseline data collection
Perinatal information: Data on the birth history and neonatal course (eg, gestational age, birth weight, sex, multiple birth status, cranial ultrasound findings, proven or suspected necrotising enterocolitis, maternal antenatal corticosteroid administration, postnatal corticosteroid use and use of oxygen at discharge from hospital) will be collected by the research team members from medical files and the hospital neonatal database. Prior to randomisation, consenting parents will be asked to complete baseline questionnaires to obtain the following information:
Social risk: The Social Risk Index which assesses six aspects of social status including family structure, education of primary caregiver, primary income earner occupation, primary income earner employment status, language spoken at home and maternal age at birth will be used to assess social risk.44
Parenting self-efficacy: The Karitane Parenting Confidence Scale is a reliable and valid measure for assessing parent confidence in 15 task-specific areas including confidence in feeding, settling and decision-making.45 This will also be collected at 12 and 24 months’ CA.
Parent depression, anxiety and stress: The Depression Anxiety and Stress Scale 21 evaluates the severity of symptoms associated with depression, anxiety and stress and categorises results as mild, moderate or severe.46 This will also be collected at 12 and 24 months’ CA for all participants, and at 3 and 6 months’ CA for parents in the intervention group.
Parent quality of life: The Short-Form Six-Dimension Quality of Life instrument will be used to facilitate the estimation of quality-adjusted life years and to inform the cost-effectiveness of the intervention.47 This will also be completed at 12 and 24 months’ CA.
Use of therapy services: A purpose-built questionnaire will be used to measure access to therapy services for the child. This will also be administered at 3, 6, 12, 18 and 24 months’ CA to monitor access to early intervention services (see online supplemental material 2).
Supplemental material
Randomisation
Following completion of all baseline questionnaires, infant participants will be randomised to the TEDI-Prem intervention or usual care group in a 1:1 ratio when the infant is ≥32 weeks’ gestation and medically stable. Randomisation will be computer generated using block randomisation with variable block sizes and stratified by site of recruitment and multiple births. Infants from multiple births will be randomised to the same group due to the nature of the intervention. Randomisation will be conducted using a web-based randomisation programme to ensure allocation concealment. Following randomisation, parents will be notified of group allocation by a research team member. All participants will be gifted four age-appropriate toys at the baseline assessment as a thank you for their participation in the trial, and will receive a support services information letter with contact and referral information for different support options in the community.
Trial intervention
In addition to usual care, participants randomised to the intervention arm will receive the TEDI-Prem intervention programme which will start while the infant is in the hospital. See table 1 for an overview of how TEDI-Prem differs from usual care. The intervention programme is a collaboration between a physiotherapist/occupational therapist (TEDI-Prem therapist) and the parent. It consists of thirteen 45–60 min sessions delivered across three phases, from infant randomisation up until 12 months’ CA. Due to the nature of the intervention, the participants and TEDI-Prem therapists will not be blinded to the trial intervention. Four TEDI-Prem therapists will be involved in administering the intervention throughout the trial.
The novel intervention is grounded in neurorehabilitation and parent–child interaction theory and combines a model of care where there is continuity of care from the hospital into the community environment by using telehealth and web-based education modules which can be adapted according to the needs of the individual infant and family. As per the SPEEDI study,34 the TEDI-Prem intervention uses a perception action model of development whereby an infant’s motor activity supports their attempts to explore and engage with their environment, allowing the infant to receive and interpret important information and solve problems by linking the mind and body in a cycle that supports development across multiple domains.48 Further, the TEDI-Prem intervention uses the theoretical approaches from e-prem which was designed to work with parents to support their mental health and relationship with their child to improve child development. Consistent with Bronfenbrenner’s bioecological theory of development, the parent–child relationship provides the child’s most proximal, strongest and immediate environment for development49 and many of the positive effects of early intervention programmes for preterm children work through improving the parent–child relationship and parent adaptability.50 Belsky’s determinants of parenting model also highlights how factors such as parent mental health influence parenting.51
The TEDI-Prem intervention content and strategies were developed to address the deficits commonly seen in infants born preterm and their parents,52–54 and include using self-calming strategies and environmental support to enhance parent–infant interaction, with large doses of practice to support postural control and learning, visual motor and object interactions in the first few months of the intervention, along with psychosocial education to support parent mental health and well-being. The TEDI-Prem therapist uses the key strategies outlined in box 3 to address the principles of the programme and increase the infant’s opportunities for movement.
Telehealth for Early Developmental Intervention in babies born very preterm key strategies
Providing graded postural support;
Observing spontaneous movement in response to support;
Varying postural support to encourage different opportunities and sensorimotor exploration;
Varying positions with minimal support to encourage variable, infant-directed movement; and
Providing opportunities to visualise, track and manipulate objects.
As parents deliver the intervention rather than a therapist, a higher intensity of intervention can be provided to drive neuroplasticity. Throughout the TEDI-Prem intervention programme, parents learn the necessary skills to scaffold their infant’s learning. In phase 1 of the programme, there is a strong focus on guiding parents to understand behavioural cues and how to identify ideal times for play and interaction with their infant. Enhanced parental capacity for engagement and self-efficacy allows parents to transition into providing activities that support their infant’s developmental function (phases 2 and 3) and provide opportunities for early problem solving, and is also likely to have a positive impact on parental well-being.22 In phases 2 and 3 of the programme, delivered via telehealth, parents are encouraged to complete five activities (watching people and toys, tummy time, holding head up, kicking play and toy play with hands and legs in the middle), individualised to the infant’s abilities, to support variable practice with developmental play, provide environmental enrichment through positioning, presenting toys and social engagement, while supporting the infant’s self-directed movements and interactions without imposing movement.
The use of video-based telehealth sessions will allow therapists to observe the infant and work with the family (through the use of guided participation) on intervention strategies individualised to their infant and home environment.
There are several resources used to support the intervention sessions including a series of parent education handouts and videos, and an ‘Activities for Home Play’ booklet. The parent education handouts cover a variety of topics including psychosocial education to support parent mental health and well-being, education on infant development, including language and communication and education on parent–infant relationships and sensitive parenting behaviours (see online supplemental material 3 for list of parent education handouts). The parent education videos support and reinforce the content provided in the parent education handouts and demonstrate examples of the TEDI-Prem principles and five intervention activities. The ‘Activities for Home Play’ booklet includes illustrations and written instructions on the developmental play goals and intervention activities the parent and TEDI-Prem therapist will work on throughout phases 2 and 3 of the programme. Parents will be encouraged to use the toys gifted to them at the commencement of the trial during the intervention activities. Compliance and dosage will be assessed by parent report during each TEDI-Prem session.
Supplemental material
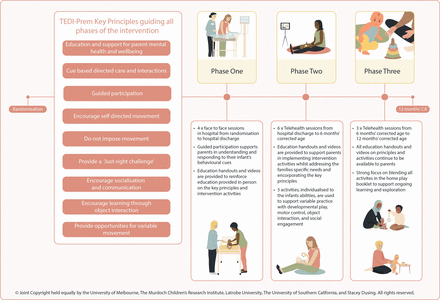
The parent education handouts and accompanying educational videos made available to parents at each phase will be individualised to ensure it is relevant to the infant’s family structure and supports the needs of the parent as they change and/or evolve throughout the programme. The TEDI-Prem therapist will make relevant resources available to parents after each intervention session via email and access to a secure, password-protected video sharing platform. New content will be provided during each phase of the programme and content from previous phases will be reviewed as required. The ‘Activities for Home Play’ booklet will be introduced and provided to parents at the end of phase one in preparation for use in phase 2, when the infant is discharged home. During the intervention sessions, the TEDI-Prem therapist will view educational videos with the parent—selecting videos based on the developmental needs and progression of the individual infant. figure 2 provides an overview of the TEDI-Prem intervention.
Telehealth for Early Developmental Intervention in babies born very preterm intervention overview. CA, corrected age.
Key principles and fidelity
The key principles of the intervention are outlined in box 4 and are the foundation from which the intervention content and strategies are delivered. These key principles will form the evaluation of the fidelity of the intervention and will be in part assessed on therapist adherence and measured by the frequency with which the intervention therapist demonstrates, talks about or brainstorms with a parent about the TEDI-Prem principles and strategies. One of the four sessions in phase 1 and all sessions in phases 2 and 3 will be video recorded. TEDI-Prem therapists will complete a self-assessment of adherence using a fidelity checklist following each session. The author’s SD and SB will randomly select three video-recorded sessions (one from each phase) per participant to further assess therapist adherence and provide feedback and additional training and/or support as required.
Telehealth for Early Developmental Intervention in babies born very preterm (TEDI-Prem) key principles
Principle 1: education and support for parent mental health and well-being
This principle aims to provide a protective influence for the development of very preterm infants.
The TEDI-Prem therapist checks in with the parent regarding their emotional health during each of the TEDI-Prem sessions. A series of supporting educational handouts and videos are provided throughout the 12-month intervention programme which focus on psycho-education and strengthening the parent–child relationship. Parent mental health is screened throughout the intervention programme enabling more targeted intervention and assisting parents in accessing support services where indicated.
Principle 2: cue-based directed care and interactions
Parents learn to read their infant’s behavioural cues during caregiving, play and social interaction. The parent learns to match their behaviour and interactions to meet the infant’s demonstrated readiness. This key principle encourages sensitive and responsive parenting behaviours and supports parents in identifying the infant’s alert and active times for intervention, developing a routine for interaction, as well as following the infants lead on when to provide rest breaks. Parents are encouraged to use vocalisation along with social and motor interactions in response to the infant’s cues.
Principle 3: guided participation
This principle builds the foundation for intense early intervention and aims to support a less skilled person in developing a specific new practice. The basic processes of guided participation that are used in the intervention are:
Providing bridges from the known to the new;
Choosing and structuring learning activities;
Structuring responsibility in joint problem-solving; and
Transferring responsibility for managing activities.
Using guided participation, the TEDI-Prem therapist assists parents in learning to read their infant’s readiness cues for caregiving, interaction and play and match their own interaction to the infant’s readiness.
Principle 4: encourage self-directed movement
This principle allows the infant ample time to elicit self-directed movement, to make errors and to correct these errors as independently as possible. Rather than aiming for a predetermined ‘correct pattern’ of movement, the infant’s own strategies for movement are supported to emerge.
Principle 5: do not impose movement
When the infant requires assistance to transition between postures, to maintain postures or to interact with objects, there is a focus on providing the least amount of assistance required and not imposing movement.
Principle 6: provide a ‘just right’ challenge
The ‘just right challenge’ is about matching the skill set required for an activity with the performance capacity of the infant engaged in the activity. Through scaffolding, that is, offering just the right help at just the right time in just the right way, the TEDI-Prem therapist teaches parents to determine and use the ‘just right challenge’ during all interactions. For infants to make continual gains in a safe, timely and positive manner, it’s important to know how much assistance they need to perform an activity and to understand ways to adapt the activity to place it at the cusp of an infant’s developmental ability. This can involve altering the level of assistance provided, changing the infant’s posture, modifying the environment and adapting aspects of the task such as object placement. The parent learns how to provide graded postural support and observes their infant’s spontaneous movement, social engagement and vocalisations in response to the support to determine if the ‘just right challenge’ has been met.
Principle 7: encourage socialisation and communication
Across postures and with varying level of postural support, parents encourage infants to focus on their faces and engage in early social interaction including vocalisations and visual engagement. Infants learn to read their parents’ behavioural cues and develop an understanding of facial expressions and vocalisations as a form of communication. As infants fix on their parents faces, smile and/or vocalise and parents respond in a timely and sensitive way, infants learn reciprocity and the value of making a sound. This provides the building blocks of social engagement and early language and communication.
Principle 8: encourage learning through object interaction
Across a variety of positions and with graded postural support, infants are provided opportunities to see, feel, mouth and hold objects that vary in weight, size, texture, hardness and colour. Consideration of object placement motivates infants to move through different positions to interact with objects. This principle enables varied cognitive and movement opportunities as well as sensory inputs. Infants learn about object affordance, cause and effect and means end as they move their body and interact with objects and the world around them.
Principle 9: provide opportunities for variable movement
Varied exploratory movements are essential building blocks for learning any new postural skill. Trial and error with movement is essential in the learning process.
*Table adapted from Supporting Play Exploration and Early Development Intervention key principles, as published previously.34
Outcome measures
At 12 and 24 months’ CA, all infants will be assessed by an examiner who is blinded to group allocation. The outcome measures in this trial and their timing of collection are described in box 5. An overview is provided in figure 3 below.
Primary and secondary outcome measures
Primary outcome
Infant Motor Composite Score on the Bayley Scales of Infant and Toddler Development-fourth edition (Bayley-4) at 12 months’ corrected age (CA).56
Secondary outcomes
Infant
Child cognition assessed using the Bayley-4 cognitive composite scores at 12 and 24 months’ CA.56
Child language assessed using the Bayley-4 language composite score and receptive and expressive scaled scores at 12 and 24 months’ CA.56
Child motor development assessed using the Bayley-4 Motor Composite Score at 24 months’ CA.56
Child behaviour assessed using the Infant Toddler Social Emotional Assessment at 12 and 24 months’ CA.57
Parent
Parental depression and anxiety assessed using the Depression Anxiety Stress Scales at 12 and 24 months’ CA.46
Parenting self-efficacy assessed using the Karitane Parenting Confidence Scale at 12 and 24 months’ CA.58
Parent–infant interaction assessed using the Emotional Availability Scale at 12 and 24 months’ CA.59
Cost-effectiveness of Telehealth for Early Developmental Intervention in babies born very preterm compared with usual care (to be published separately from the main trial results).
Costs assessed using the cost of the intervention and children’s healthcare utilisation.
Quality-adjusted life years (QALYs) assessed based on child and parent quality of life.
Child quality of life measured via parent report using the EuroQol Toddler and Infant Populations at 12 and 24 months’ CA.60
Parent quality of life measured using the Assessment of Quality of Life at 12 and 24 months’ CA.47
Cost-effectiveness of the intervention compared with usual care assessed as cost per additional QALY gained.
Outcome measures and timing of administration throughout the trial. CA, corrected age; TEDI-Prem, Telehealth for Early Developmental Intervention in babies born very preterm.
Statistical analysis
The statistical analysis will be conducted by a statistician who will remain blinded until the end of the trial. Data will be analysed using the intention-to-treat principle and will include all participants according to their treatment allocation irrespective of whether they received any of the intervention (eg, treating all intercurrent events using a treatment policy strategy). Sensitivity analysis will be conducted using a hypothetical strategy for adherence, where adherence will be defined as participating in at least 80% of TEDI-Prem intervention sessions for participants in the intervention group.
Mean differences between groups in the primary outcome will be examined using linear regression, fitted via generalised estimating equations to account for multiple births and adjusted for the site. Differences between groups in secondary outcomes will be examined using linear regression for continuous outcomes and logistic regression for binary outcomes. All models will be fitted via generalised estimating equations to account for multiple births and adjusted for the site.
Analyses will be repeated in subgroups according to social risk (high vs low), gestational age (extremely preterm vs VPT) and later neurological diagnosis (yes vs none) at 12 months’ CA via the inclusion of an interaction term in the regression models.
Patient and public involvement
We have included stakeholder involvement at several stages in the development of the trial. As part of our Centre for Research Excellence (CRE) in Newborn Medicine, we completed a Delphi study identifying the research priorities of parents with experience in newborn medicine.55 Parents identified many questions as high-priority with primary areas related to supporting parental mental health, relationships between parents and neonatal clinical staff (including involvement in care and communication), bonding and the parent–child relationship and addressing long-term impacts on child health and neurodevelopment. These consumer-identified research priorities were integrated into the design of the TEDI-Prem intervention programme and its effect on outcomes in these areas will be evaluated.
In addition, members from the CRE in Newborn Medicine’s Consumer Advisory Group (CAG) were actively involved in the development of the TEDI-Prem intervention programme and the trial’s research methods and design. Members of the CAG participated in a focus group where the consumer perspective on the appropriateness, acceptability and fit of our study name, the intervention’s key principles (underlying mechanisms of change) and forms (activities embedded into the intervention that will be used to carry out key principles, including their timing and frequency) was provided. This feedback was incorporated into the study design and procedures outlined. Further, members of the CAG reviewed and approved various research materials to ensure they are easily accessible to consumers.
Ethics and dissemination
A Data Safety Monitoring Board (DSMB) will be established to review and evaluate study data on adverse safety events, study conduct and progress and trial efficacy. The DSMB will monitor trial efficacy through a single interim analysis of the primary outcome once 50% of participants have completed their 12-month CA follow-up. The Haybittle-Peto boundary stopping rule will be applied to the results of the interim analysis to decide if the trial should be stopped prematurely. If the interim analysis shows a p value of ≤0.001 that a difference as extreme or more extreme than that found between treatments if the null hypothesis were true, then the trial will be stopped early. If additional psychological support for parental mental health is indicated, they will be provided with referral/information about appropriate support services, with their permission.
Trial findings will be disseminated through presentations at national and international conferences, publication in peer-reviewed journals, as well as digital and print media. Further, we will disseminate our research results to trial participants, individuals with lived experience of preterm birth, health professionals and service providers. This will be accomplished through direct communication with participants, collaborations with preterm parent support groups using their social media and web platforms, the CRE in Newborn Medicine’s professional networks and conference presentations. If found to be effective, training courses on TEDI-Prem will be rolled out using this protocol including the online training for therapists. Consent from trial participants to be contacted for future follow-up studies will be sought.
Discussion
This paper outlines the protocol for the trial of an early intervention programme designed for VPT infants over the first year of life. Publication of protocols enhances the transparency of research and allows for replication. The TEDI-Prem programme commences in the hospital prior to discharge and provides an intervention delivered earlier than traditional health service models of early intervention for preterm children. We propose that providing targeted intervention to support the development of the parent–child interaction, an enriched environment, promoting infants’ self-directed movement and their parent’s well-being at an earlier age while in the hospital and across the first year will lead to improvements in neurodevelopment, parental mental health and a cost-effective model of delivering early intervention services for VPT infants compared with usual care.
Ethics statements
Patient consent for publication
Acknowledgments
We would like to acknowledge and thank the following: Alex Aldis, Consumer Advisor and Associate Investigator for his guidance and critique of the conception and design of the study. The members of the Centre for Research Excellence in Newborn Medicine Consumer Advisory Group for their input into the study design and revision of study and intervention materials.
References
Footnotes
X @kmdalziel, @aliciaspittle
Contributors SD, PJA, KD, AEH, RWH, KL, ATM and KT were involved in the conception and design of the study. ALE and AJS were involved in the conception and design of the study, and initial manuscript preparation. All authors provided a critical review of the protocol, approved the final version as submitted and agreed to be accountable for all aspects of the work. AJS is the guarantor.
Funding This work is supported by the Medical Research Future Fund (MRFF) administered by the Australian Commonwealth Government through the Department of Health (Funding Application No: MRF1199780).
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.