Article Text
Abstract
Objectives To understand the competitive position of the UK in comparison to Europe and the USA for haematological cancer clinical research.
Design Using commercially available databases, clinical trial numbers, their effectiveness and publication outputs were evaluated in two analyses: a macrodevelopment and a research activity and performance analysis.
Data sources The following databases were used for this analysis: Organisation for Economic Co-operation and Development, Thomson Reuters Incidence and Prevalence, the Cortellis Clinical Trial Intelligence, the Clarivate Cortellis Innography Patent Intelligence, Thomson-Reuters Cortellis Regulatory Intelligence, Thomson Reuters Web of Science and data from the Centre for Medicine Research (CMR).
Eligibility criteria European countries with comparable geography, healthcare standards and economies, as well as the USA, the largest country where research is conducted. All haematological oncology clinical trials from phase 1 to phase 3 were included.
Data extraction and synthesis All data were retrieved in September 2017 and macroeconomic data were reviewed in 2022; haematological cancer data were restricted to leukaemias generally as a surrogate reference for haematological oncology indications; research output publication data were evaluated using specific MeSH/keyword search terms between 2010 and 2017. Key metrics explored included healthcare expenditure per capita, study experience across countries, comparative capability of each country for clinical trial implementation, clinical trials’ performance and impact of research as measured by impact factor and citation metrics of publications.
Results Revenue for clinical studies is lower in the UK than European comparators. All studied countries had comparable leukaemia prevalence rates, but the UK spent least per capita on healthcare versus France, Germany, Spain and the USA. The number of clinical studies in the UK showed a decline compared with other European countries. Clinical trial implementation was lowest in the UK (n=380) versus Germany (n=665), France (n=643), Spain (n=632), Italy (n=538) and the USA (n=3254). Registered versus active clinical studies suggested the USA had the highest number underway (n=824), with the UK ranked fourth of five European countries (Germany=239, Italy=232, France=211, UK=177 and Spain=141). However, the UK had the highest completion rate of phase 3 studies it did initiate (n=154, 87%) and performance was comparable with Germany (n=188, 78.7%) and France (n=151, 71.6%). When analysed by phase, the UK was the second highest European performing country (n=121) for phase 2 study completion compared with Germany (n=182) both less than the USA (n=345). The UK completed the most phase 1 studies compared with other European countries, only second to the USA (n=31 vs n=126). However, the UK clinical trial performance metrics were negatively impacted for the UK compared with other European countries with respect to clinical trial application (CTA) process, timelines, ethics committee approvals, median time to start up and rate of non-enrolling sites. The UK was slower to initiate studies (median 186 days) vs Germany (92 days), France (141 days), Italy (122 days) and only marginally faster than Spain (195 days). While median enrolment rates were comparable across all countries, the UK had the highest proportion of sites that failed to enrol any patients (despite regulatory timings being comparable to Germany (90 days) and France (95 days)). However, publication of data following clinical trials in the UK was robust and of the highest quality compared with other countries, judged by journal placement and publication citations. The UK published high-quality, diverse research with citation rates (11.8) from clinical studies which was higher than every other country, including the USA who published fivefold more publications per year.
Conclusion While research in the UK remains among the highest quality and value globally, the UK may be losing its position globally as an attractive destination for executing clinical trials. This may be a trend that is recognised by the UK Government, but it is vital to reverse the trend of clinical trial decline and to improve the economic outlook for the UK and patient early access to innovative cancer medicines.
- Leukaemia
- Health Equity
- Clinical Decision-Making
Data availability statement
Data are available on reasonable request. All data are available on reasonable request to the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The strengths of this study are the use of robust commercial databases that give an accurate overview of clinical trial volumes in the UK and reference countries, including a breakdown of clinical trials by phase and data relating to enrolment.
Limitations include the timing of data analyses which cannot explore data in the years up to and following the signing of the Brexit exit agreement at the end of 2021 and is being explored in ongoing research.
Another limitation is the assumption that publication in the UK reflects the prestige of UK researchers and work undertaken in the UK; the impact of publication in the UK because of other factors was not evaluated.
Introduction
Clinical trials are critical to provide early access to life-saving medicines for patients, improve future standards of clinical care and inform future clinical practice that optimises patient outcomes.
Development of new therapies is expensive with costs estimated from US$765.9 million to US$2771.6 million depending on the indication.1 Clinical trials by the pharmaceutical and biotech industries are an essential part of the clinical trial mix of industry and academic sponsored studies which support clinical development of new therapeutic options to advance patient outcomes. Cost and complexity of development of novel molecules and clinical trial infrastructure mean that industry and academic collaboration in clinical trials is critical.
Industry-sponsored clinical trials provide material economic benefits to healthcare in the UK through revenue generation. Clinical trials, regardless of their funding, also provide opportunities for patients to access novel medicines and resources for the duration of the trial and maybe beyond into long-term extension phases. In Italy, 92% of funding for clinical studies was derived from pharmaceutical industry, quantified at more than €750 million.2 It was further suggested that industry-sponsored clinical trials also further benefit country healthcare systems in terms of avoided costs, whereby patients enrolled on clinical trials reduce financial burden on healthcare systems for the duration of the clinical trial.3 Two recent studies have suggested that for every Euro invested by the pharmaceutical industry, there is a net financial gain for the overall economy of at least 25%.4 5 A study in Austria suggested additional positive outcomes of clinical trials including employment of specialised staff including researchers, medical and managerial personnel so that clinical trials provide an ‘employment multiplier’ of 1.66,4 a benefit that may also be realised in other geographies where clinical trials are carried out.
Clinical trials enable clinicians to provide treatment within the trial setting without charge and often subsidise care through free diagnostics and other treatments and provide funding for administrative and operational work. Additionally, it ensures that physicians remain at the cutting edge of scientific advances as they get first-hand experience in applying the newest scientific advances.
Clinical trials provide benefits to both clinicians and patients. For patients, trials provide access to innovative and potentially life-prolonging medications years in advance of regulatory approval and reimbursement. For clinicians, clinical trials enable participation in cutting-edge medical research that can improve and shape current and future standards of care. However, for patients, it must be noted that access to innovative, experimental treatments is not risk-free, and participation in such trials runs a risk of experiencing adverse events, possible toxicity or lack of drug effectiveness.
In areas such as oncology, access to innovative medicines remains an area of high unmet need. This unmet need is recognised by the UK Government. A recent UK Commons Cancer Services report April 2022 stated: ‘Despite progress, UK patients still have much worse 5-year survival rates for many cancers than those in similar nations. Early diagnosis and fast and equal access to the latest treatments for all patients is key to reversing poor trends in National Health Service (NHS) cancer care’.6 The outcome for UK patients relies on a complex interchange between available infrastructure, access to therapies and standard of care. This report does not fully clarify, nor was it evaluated, whether poorer patient outcomes in the UK are linked to limited opportunities to participate in clinical trials. However, this remains an important aspect of the treatment journey that warrants further investigation. Clinical trials do provide an avenue for patients to access novel medications so it follows any impact on clinical trial start up, implementation and delivery may impact patient access and maybe outcome. However, it is likely to be minor compared with issues of NHS waiting lists that can impact cancer pathways for patients.
The attractiveness for a country to implement and deliver clinical trials relies on a combination of factors including market size, regulatory timelines, costs of implementation and clinical trial outputs. Data from the Association of British Pharmaceutical Industry (ABPI) suggest that despite applications for clinical trials to the UK Medicines and Healthcare Regulatory Agency (MHRA) remaining stable, the number of industry-sponsored studies has declined over the course of 2017–2020, reducing from 667 to 508, a decrease of 24%.7
Now that the UK has left the European Union, exploring the potential for the UK as a major player in clinical trials is essential. Brexit poses additional challenges to the UK in attracting clinical trial implementation including barriers to data sharing, the removal of regulatory mutual recognition, compatibility of regulatory and medicine research approaches, information access and health security.8
In this analysis to explore the attractiveness of a country for implementation of clinical trial programmes, the following points were considered:
Country population.
Expenditure on healthcare per capita.
Prevalence of disease.
Clinical trials experience.
Clinical trial and ethics approval processes and timelines.
However, understanding exclusively the number of clinical trials within a country is to ignore the scope and value of research being done. To address this, an analysis of clinical trial performance and calibre of research by publication and patent applications was undertaken, to give an overall view of not only research volume but also its value. This is imperative because while clinical trial volume may be of significant financial benefit to a healthcare economy, it is the scientific value that is important to progress new therapies for patients and improve outcomes.
The aim of this research was to explore and understand the attractiveness of the UK as a destination for running clinical trials compared with reference European countries and to understand the requirements for change to ensure the UK can become and remain an essential and attractive country for clinical trial development and implementation. This research also compared European and UK data against that for the USA as a high-income country with comparable healthcare standards. This research provides a snapshot of the UK’s standing versus key European countries and the USA, using haematological cancer as an example, with respect to clinical trial development, implementation and regulatory approval.
Methods
Countries across Europe were included in this study (France, Germany, Italy and Spain) as comparators for the UK. These countries were evaluated as all were considered high-income countries with comparable healthcare standards and economies, geography and socioeconomic factors. The USA was also included as a comparator high-income country with a robust approach to research, comparable to, but different from Europe.
Data to explore country wealth and healthcare expenditure, disease prevalence, clinical trial implementation and publication of clinical trial data were collated. Country economic data were established using data from the Organisation for Economic Co-operation and Development (OECD Paris, France), and while healthcare spend cannot always provide insight into the quality of healthcare delivery, countries were chosen for their approximate comparability. Data for disease epidemiology were provided from the Thomson Reuters’ Incidence and Prevalence Database (IPD, Clarivate, Philadelphia, USA). The IPD covers over 4500 diseases, procedures, symptoms and other health issues for incidence, prevalence, morbidity, mortality, comorbidity, treatment or diagnosis rates and cost. The IPD tracks and reports the epidemiological content from more than 280 medical journals and over 35 government and industry agencies. It is updated every 4 weeks and so is an efficient single source to access current data. Limitations of the databases used meant it was not possible to evaluate specific haematological cancers (leukaemia, multiple myeloma, relapsed refractory myeloma, non-Hodgkin’s lymphoma, diffuse large B-cell lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma, cutaneous T-cell lymphoma, follicular lymphoma and CNS lymphoma) and so leukaemia was adopted as a surrogate for all haematological cancer subtypes across the countries studied. Clinical trial numbers and stage of development were evaluated using data extracted from Cortellis Clinical Trial Intelligence (extraction date September 2017) (Clarivate). To analyse the number of completed haematological cancer studies within development phases 1, 2 and 3 for each of the six countries, Clarivate Cortellis Clinical Trial Intelligence was searched. A modified Preferred Reporting Items for Systematic Reviews and Meta-Analyses analysis approach was used to identify all studies that had been conducted within the haematological cancer disease area in each of the countries. The Cortellis Clinical Trial Intelligence database is a single source of fully searchable and indexed global clinical trial content for more than 400 000 clinical trials evaluating drugs, drug-like entities, treatment/prophylactic medical devices, biomarkers, biologics and vaccines. Data are populated daily from thousands of global sources, including more than 25 international clinical trial registries (including ClinicalTrials.gov, EudraCT, ISRCTN, JapicCTI etc), press releases, literature articles and conference reports.
To evaluate the value of clinical studies as well as volume, measures such as trial completion, published data and patent applications leading to possible useable drug entities were explored using Thomson Reuters Cortellis Regulatory Intelligence and Thomson Reuters Web of Science (WoS) (both Clarivate). The Thomson Reuters Cortellis Regulatory Intelligence is a single, comprehensive source for global regulatory information and contains more than 143 000 current and historical documents from over 200 regulatory sources covering more than 70 countries. Data relating to the CTA and the site ethics submission process and estimated duration were extracted from Cortellis Regulatory Intelligence for each of the six countries.
MeSH and keyword search terms for both American and British English were used. Parameters for publication searches were “Leukemia” OR “Leukaemia” OR “Myeloma” OR “Myelodysplastic syndrome” OR “Myelodysplasia” OR “Myelofibrosis” OR “Myeloproliferative neoplasm” OR “Hematological malignan*” OR “Haematological malignan*” OR “Hematological cancer” OR “Haematological cancer” OR “Blood cancer” OR “Bone marrow cancer” OR “Plasma neoplasm” OR “Plasma cell neoplasm” OR “Plasmacytoma” OR “Liquid tumor” OR “Liquid tumour” OR “Hodgkin* " OR “Waldenstrom macroglobulinemia” OR “Reed-Sternberg cells” OR “Lymphoma” OR “B cell neoplasm” OR “T cell neoplasm” OR “Natural Killer cell neoplasm” OR “NK cell neoplasm” OR “Heavy chain disease” OR “Lymphomatoid granulomatosis”.
Performance of clinical trials as a method to establish the attractiveness of a country for clinical trials was evaluated using data from the CMR (Clarivate) and the metrics of trial start up, trial site recruitment rate and clinical trial site efficiency. The CMR database contains biopharmaceutical company sourced and validated performance metrics for over 400 000 sites and over 25 000 sponsor-led, investigational drugs trials and is considered a gold standard for clinical performance metrics across the pharmaceutical industry. Trial start-up was measured as median time from the end of the CTA approval process to enrolling the first patient. Recruitment rates were assessed by the number of patients enrolled per site per month. Evaluation of site efficiency was measured by the proportion of sites which were initiated to start trials but did not enrol any patients. Patents for clinical medicines in haematological cancers were analysed using Thomson Reuters WoS and Thomson Reuters Cortellis (including Derwent World Patents Index) databases. These databases were selected as they contain multidisciplinary content covering 12 000 of the highest-impact journals and 150 000 conference proceeding papers, as well as data on product pipelines sourced from press releases, company reports, industry deal reports and conference abstracts.
Patient and public involvement
No patient or public involvement was required in this analysis.
Results
Analysis of countries studied
Country wealth and healthcare expenditure
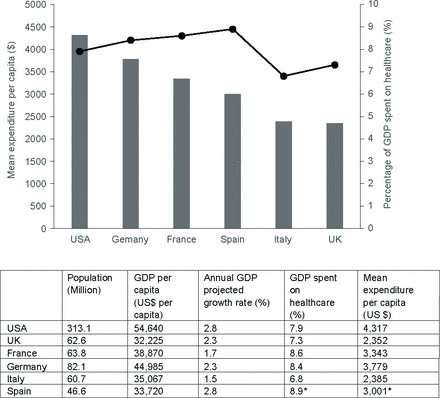
Results from the macrodevelopment analysis indicated that population size and healthcare spending were not proportional. The highest population from OECD data was observed in the USA (313.1 million) with a Gross Domestic Product (GDP) percentage spend of 7.9%. These data compare with Germany whose population at 82.1 million is a third of the USA but spends 8.4% of its GDP on healthcare. For both countries, GDP growth rates were comparable (figure 1). GDP spent on healthcare as a proportion of mean expenditure per capita was lowest in the UK at less than 5% (figure 1). Data for Spain were not available from the OECD but indicated a GDP spend of 8.9%—the highest of all countries evaluated with a population of 46.6 million.
Overview and proportion of GDP spend on healthcare by capita across study countries. Data from The Organisation for Economic Co-operation and Development 2017 country summaries. *Data were not available for Spain when the search was conducted, and subsequently checked in 2022. GDP, Gross Domestic Product.
Investment in healthcare suggested the highest expenditure in the USA with the UK and Italy the lowest investing countries per capita (figure 1). USA healthcare expenditure per capita was almost double that of the UK (US$4317 vs US$2532). Comparison of GDP per country and the contribution to global GDP was noted as France GDP US$3533 billion, Germany GDP US$5,011, Italy GDP £3180 billion and the UK whose GDP was US$3479 billion all of whom contributed 2% to global GDP and Spain GDP US$2301 billion of which 1.3% was contributed to global wealth. Healthcare spending in Germany, France and Spain was comparable (range 8.6%–8.9% GDP). When mean expenditure on healthcare per capita was examined the UK ranked the lowest of all countries evaluated (figure 1).
Disease prevalence across comparator countries
The prevalence of leukaemia across all countries in the study was broadly comparable (table 1), ranging from 32.3/100 000 population in the UK to 45.6/100 000 population in France. As expected, the USA, with a population size more than three times larger than the largest European country Germany, the estimated total number of patients with leukaemia was greater than 100 000. The UK had a projected leukaemia population equivalent to Italy and more than 9000 patients fewer than both Germany and France.
Prevalence of leukaemia* data by country and estimated number of total patients
Evaluation of clinical studies across comparator countries
Implementation of clinical trials from phase 1 to phase 3 registration trials
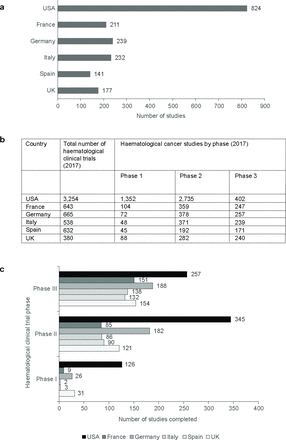
The number of active haematological cancer trials underway was evaluated against the number of registered studies. In 2017, the number of active haematological cancer clinical studies was lowest in both the UK and Spain (n=177 and 141, respectively) compared with all other countries (figure 2a). The UK lagged considerably behind Germany, Italy and France, despite having a comparable population size and mean healthcare expenditure spend to Italy.
(a) Number of active haematological cancer trials in study countries; (b) documented number of clinical trials by phase by country; (c) completion of clinical trials by phase by country.
Most haematological cancer trials were conducted in the USA (n=3254) with the UK and Italy having the lowest number of trials (n=380 and 538, respectively) (figure 2b). The distribution of these studies by country and phase is shown in figure 2 and demonstrated a predominance of phase 1 and phase 2 haematological cancer studies in the USA (figure 2c). The UK conducted a comparable number of phase 1 haematological cancer studies with other European countries (figure 2b). Phase 2 clinical trials were the most commonly implemented trial phase in the UK but the number of phase 2 studies was lower than Germany, France and Italy who conducted the most phase 2 haematological cancer studies outside of the US (figure 2b).
The number of phase 3 haematological cancer studies was broadly comparable across Europe (n=239–257) except for Spain which conducted only 171 phase 3 studies. The UK conducted 240 phase 3 studies and ranked third among European countries suggesting that the UK performs well with respect to this phase of study (figure 2b). As expected, European figures were considerably lower than the number of phase 3 studies conducted in the USA (n=402) (figure 2b).
Clinical trial volumes were evaluated through exploration of the number of clinical trials completed by phase and country (figure 2c). These data suggest that while clinical trial volume in the UK may be low in comparison to other European countries, once initiated clinical trials in the UK are very likely to be completed. For phase 2 and 3 studies, the UK was third of all comparator countries completing more studies behind only the USA and Germany, and for phase 1 studies, the UK was the highest European country completing studies with only the USA completing more.
Not surprisingly, the number of clinical trials completed in the USA was comparable to the sum of all European countries considered in this study. The USA had the highest rate for trial completion across all phases and Spain and Italy the lowest. With respect to trial completion across phases 1–2, the USA trial completion counts were at least twofold greater than any individual European country included in the analysis (USA n=728; Germany n=396; UK n=306; France n=245; Spain n=225; Italy n=226). Study completion by phase for USA was fourfold higher for phase 1. For phase 3, clinical studies USA rates of trial completion were higher but less pronounced than overall data (30%–50% higher than European countries) and the USA completed approximately 27% more studies than Germany, the highest-ranking European country. In phases 2 and 3, the UK was the second highest completing country in Europe outside of Germany.
Analysis of clinical trial effectiveness
Clinical trial implementation
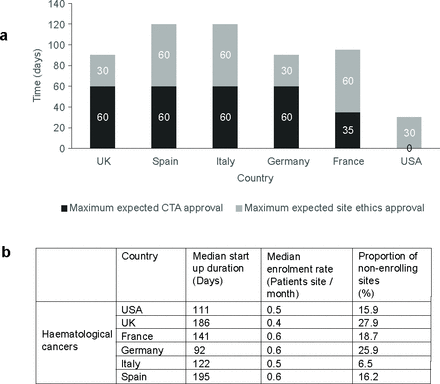
Clinical trial implementation and completion provide an insight into research across comparator countries but do not provide insight into the potential impact and effectiveness of clinical trials and their outcomes in those countries. A review of CTA and ethics approval times revealed that they were demonstrably shortest in the USA (figure 3a) for national review and approval. Spain and Italy had the longest processes at 60 days for CTA approval and 60 days for ethics approval. For the UK, ethics’ approval times were a 30-day maximum and CTA approval maximum of 60 days and this was the same as that for Germany (figure 3a).
(a) Maximum expected approval times for clinical trial applications and ethics approvals; (b) clinical trial performance metrics by countries evaluated. CTA, clinical trial application.
Evaluation of clinical trial effectiveness across countries is shown in figure 3b, demonstrating that Germany was the fastest European country in clinical trial start up being twofold faster than the slowest European country, Spain. The UK was only marginally faster than Spain with a median of 186 days from approval to trial start up. The UK also had the highest rate of non-enrolling clinical trial sites at 27.9%, an attrition rate that was comparable with Germany (figure 3b), but almost twice that of the USA and four times higher than Italy. Across all countries, median enrolment rate per site over time was broadly comparable.
Analysis of clinical trial outcomes
Research impact analysis
To explore the impact of clinical trial research across the studied countries, the volume and impact of clinical trial-derived data were evaluated. Turning research implementation into outputs as measured through publications, citations and patents was highest in the USA, followed by Germany, with Spain lagging (table 2). While total publication numbers were similar between Italy and UK (11 143 and 10 579, respectively), citations derived from UK-published research were the highest of all countries evaluated (table 2).
Research output by country 2012–2017 as evaluated from publications and patents
With respect to calibre of research publication diversity, the UK was a smaller entity for clinical trials, but research was published in high calibre, highly cited journals ranking third of all countries evaluated, including the USA. Similarly, the number of patent applications arising from UK-sited clinical trials was high, ranked third of all countries evaluated and the second highest in European countries (table 2). Evaluation of research diversity in the UK with respect to haematological cancers derived from keyword representation suggested the UK has a broad diversity of research and was the only country where there was a predominance of publications in childhood leukaemia, a trend not reflected elsewhere.
Discussion
Clinical research has the potential to advance science and revolutionise outcomes for patients. There are economic, healthcare and societal benefits for the UK being part of this milieu. However, clinical trials require investment in terms of time, finance and researcher training, which is outweighed by the potential benefits both to patients and society.
Clinical trial implementation
In this analysis, the maximum expected time to achieve a CTA for each country was calculated. From this, it was clear that the UK and Germany have the fastest approval times for CTAs and ethics approvals. Slower time in other countries such as Spain (120 days vs 90 days for the UK) reflects the sequential nature of processes for a favourable opinion from the Clinical Research Ethics Committee (CREC/EC), the agreement from the management of the centre where the study is to be conducted and the authorisation of ethics committees and the Spanish Agency of Medicines and Medical Devices (AEMPS). By contrast, approval times in the USA were the shortest, but our data do not reflect any interstate differences that may arise.
However, with respect to clinical trial start up in 2017, the UK was slow compared with Germany. The difficulties in starting clinical trials in the UK have been noted and governmental interventions have been implemented to reverse this trend in the post-COVID-19 era including an increase in funding and removal of capital investment taxes for medicinal development in 2023 and the policy paper of the future of clinical research implementation plan 2022–2025 published in 2022.9 There are glimmers of hope that these initiatives are showing promise. However, with a recently elected government in place in the UK, it will be imperative that the strides made to keep the UK competitive within clinical trial implementation and delivery is maintained and enhanced.
Clinical trial performance
Our research suggests that while the UK in 2017 was performing relatively well compared with the rest of Europe, with some greater expertise in phase 1 and phase 2 studies, it is losing ground. Overall, the UK conducted the fewest haematological clinical trials, ranking fourth out of five European countries for phase 2 studies although better rankings were observed for both phase 3 and phase 1 studies. While it must be noted that differences between countries were not substantial when ongoing studies were evaluated, the UK overall was ranked fourth out of five, with only Spain conducting fewer studies in the year of investigation. This is despite the UK having similar disease prevalence and healthcare expenditure to higher performing countries like Germany. Healthcare spend per capita may not be translatable to delivery of high-quality healthcare, but when GDP percentage values for healthcare are also juxtaposed with ongoing haematological cancer trials as of 2017, the UK lagged behind almost all European countries, bar Spain, despite comparable prevalence data across all countries studied. We have conducted a second study exploring clinical study implementation over time, currently unpublished, which suggests that clinical trial numbers in the UK declined after 2017 to near 2011 levels, which is an effect not seen in comparator countries. While this companion study represents the pre-COVID-19 environment a recent report has indicated that clinical trials initiated by industry has reduced by 15% to 4900 trials in 2023 compared with 2022 and more than 32% lower than the peak in 2021, which was driven by COVID-19 trials.10 11 The authors of this report suggested a potential driver of this decline was a historic delay in trials startup or completion and that clinical trial starts in oncology, metabolic/endocrinology, immunology and neurology together were reduced by approximately 8%.10 11 Our data give a snapshot of haematological oncology trials which is consistent with other published reports in other areas of clinical research such as the report from IQVIA published in 2024.11
One element that cannot be evaluated from the database results is the sponsor of clinical trials, whether academic or industry sponsored. Recent data suggest that sponsorship of clinical trials by academic sources is robust with up to 62% of clinical trials sponsored in this way.12 Academic sponsored studies encompass a wide range of study types from the investigation of novel medicines or techniques to evaluation of real-world clinical practice, while industry-sponsored study most often focuses on the investigation of novel medicines or use of existing medicines in new therapeutic areas. However, publication of results from academic clinical trials lags behind that of industry-sponsored studies, likely due to reasons of restricted financial or personal capacity or academic priorities.13 Either way, limiting publication of data risks an overall publication bias in available data, rectification of which would benefit patients and researchers alike and demonstrate a continued need for a diverse clinical trial landscape.
In oncology specifically, a review of trials by the WHO from 2012 to 2017 indicated that 71% of trials were industry funded.14 In the UK, the ABPI report indicated a decline from 2012 in clinical trials in the UK,7 a position supported by Cancer Research UK. As we have seen, governmental interventions have been devised and implemented to reverse this trend but fledgling programmes such as this will need time, investment and governmental commitment to ensure they thrive.
Patient population sizes ranged from 17 661 in Spain to 29 093 in Germany. When pooled across Europe, the sizes were broadly comparable to those in the USA (116 036 vs 103 636). The USA conducted almost six times as many haematological cancer clinical trials compared with the UK, a trend that was replicated across clinical studies overall. In this study, the number of clinical trials in the USA was not adjusted for population so clinical trial volume was predominantly a function of population size, and this explains why the USA, with a fivefold larger population, ranks first. With such access to patients and a comparable prevalence of leukaemia in the UK and other European countries their ability to engage with eligible patients outranks individual countries in Europe. Evaluation of trial completions shows that the USA consistently ranked highest in terms of completion, and therefore, experience. These data are unsurprising given its much larger population compared with each individual European country included in the study and reflect trends in clinical trials overall.15
The highest trial completion in Europe was seen in Germany, perhaps reflecting their high levels of experience of running clinical trials as defined by this analysis. For the UK and France, the picture was a little more variable. The UK had a high rate of study completion for phase 1 clinical studies specifically, perhaps reflective of the UK’s experience in this area. Importantly, if the proportion of completed haematological cancer trials against the number of active trials was evaluated it was apparent that while numerically the UK may have implemented fewer clinical trials it does drive them through to completion; third behind the USA and Germany for phase 2 and phase 3 studies and second only to the USA for phase 1 studies. These data suggest that clinical trial research in the UK may be hindered numerically, but clinical trials started in the UK are typically completed and published with high-impact and highly cited published data.
Clinical trial effectiveness
An overview of clinical trial efficiency using proportional data provided a different perspective. As shown in figure 3, Italy, a less populated country, was most efficient in clinical trial start up, recruitment and enrolment, with the USA second and the UK fifth ahead of Spain. Data suggest that the UK has lost some impetus in initiating and driving clinical trials with long start-up times to initiate studies which will have consequences for the UK economic and for patient access to innovative medication. This trend, it is hoped, will be halted and reversed.
Approval time is a key factor in determining the attractiveness of a country for clinical trial roll out. Typically, in any country, a CTA needs to be reviewed by a number of organisations in addition to central and local ethics committee reviews. Depending on the specific country, review processes may be sequential or in parallel, with each review having an expected maximum duration. From the various country-specific processes, it was possible to calculate the maximum expected review time for a CTA. In the UK, authorisation from the MHRA is mandatory to conduct a clinical trial alongside favourable opinions from relevant ethics committees. Applications to the ethics committee and the MHRA may proceed in parallel or sequentially, depending on the wishes of the sponsor organisation. By comparison, in Spain, it is necessary to obtain a favourable opinion of the corresponding Clinical Research Ethics Committee (CREC/EC), the agreement from the management of the centre where the study is to be conducted and the authorisation of ethics committees and the Spanish AEMPS processes which may or may not proceed in parallel, but are often enacted sequentially. By contrast, it is interesting to note that with respect to clinical trial implementation the UK was ranked low, typically fifth of the six countries evaluated. At 186 days, UK authorisation took twice as long as Germany and was a third longer than the USA.
The UK is uniquely placed for clinical trial implementation by merit of the NHS whose infrastructure can provide a well-characterised patient population for studies, combined with world class medical capability. However, as a largely Trust-based decentralised system, any advantages may be diminished, and this may explain the UK rankings among other European countries. Clinical trial providers often need to undergo lengthy negotiation with the NHS before a trial can be set up, resulting in slower start up times to enrolment, higher trial costs per patient than other European countries and a mitigation of any advantages a shortened maximum time to ethical approval might give a trial sponsor. However, it is hoped that initiatives underway to improve clinical trial implementation in the UK may soon reap benefits for the UK economy and patients.
This analysis could not evaluate how many trials undertaken in the studied countries led to licensed, regulatory approved and available medications accessible to patients. However, the number of patents related to clinical trials can be surmised to be a surrogate marker for molecules that are considered promising. In Germany, the number of patent applications in 2017 was more than 1000 with 30% fewer applications lodged in the UK in the same period (n=729). This places the UK only second in Europe behind Germany which suggests that the patent environment in the UK is quite attractive. However, given that the UK is 30% behind Germany, it is tempting to speculate that Germany has more innovation-driving patent applications. However, with the UK and Germany having comparable times to ethics and CTA approval and the UK having 30% fewer patent applications Germany’s innovation alone is insufficient to explain the disparity between Germany and the UK. Going forward, it would be interesting to explore in a future study, how governmental intervention, the settling of the economic landscape post-Brexit and the renewed investment in clinical trials that have resulted following COVID-19 change the landscape for clinical trials and patent applications in the UK. The changing governmental landscape in the UK may benefit further with increased investment or political willingness and it will be interesting to evaluate the combined impact of these factors in the future. Within Europe, the Accelerating Clinical Trials programme16 is similarly purported to transform the EU clinical trials landscape and so the impact of this on the UK, as a non-EU member, is as yet unknown and this warrants further investigation.
Contrasting volume against quality as a metric for attractiveness
Volume analysis alone, however, provides only a limited perspective. Understanding the value and reach of clinical trial outcomes is of equal importance and is vital to progress therapies for future approval and use in patients. Analysis of research output and quality was used as a surrogate marker of clinical trial performance. In the UK, it was noted that clinical trials when completed were typically published in high-ranking, highly citable journals. Such wide publication of UK-led data in reputable journals demonstrates that clinical data derived in the UK is widely accepted by the academic and clinical community as being of high quality. The UK’s demonstratable drive to publish data ranked it second in Europe in terms of publication volume behind Germany and third when the USA is included. However, the UK ranks first in Europe with respect to a number of citations per publication and only second behind Germany for total citations. Consideration of publication citations, which were highest in the UK, infer that research in the UK is robust, relevant and interesting and we would translate this inference as a surrogate marker that the UK is a deliverer of high-quality research. Empirically, it might be assumed that there will be a substantial lag between the end of a clinical trial, regardless of its phase, and publication in a highly reputable journal. While this may be true in some instances and is believed anecdotally, recently published data indicated a median time from trial completion to publication of only 431 days (range 278–618) which was not statistically different for positive or negative results.17 18 Time lags in data dissemination, however, when they do arise can be acknowledged to impede knowledge diffusion, and in the case of novel medicines may impact time to regulatory approval and reimbursement which may require published data for their evaluation. However, while the publication data presented here are from 2017 and may reflect clinical data completed in the previous year or two, they remain a robust marker of high-quality data dissemination. We anticipate that this trend will continue over time, regardless of any changes to the clinical trial landscape in the UK.
Positioning the UK as an attractive clinical trial partner
The UK has demonstrated instances where academic clinical trial infrastructure can align to deliver effective, timely and impactful results. Examples of this include the RECOVERY trial evaluating approved drugs in the management of COVID-19 and the STAMPEDE trial exploring the role of radiotherapy in prostate cancer.19 20 While COVID-19 was a once-in-a-generation event, promoting a highly favourable landscape for clinical research, it shows that the UK can deliver clinical trial excellence that leads the world, particularly for the evaluation of approved therapies. However, for the UK to be a go-to destination for clinical trial research for preapproved or investigational molecules, other drivers besides research quality need to be understood, for example, clinical trial activity. Our results show that in terms of phase 3 clinical trial activity the UK is comparable to Italy, but lags behind Germany and France, a gap that is wider when phase 2 trials are considered. From a European perspective, Germany remains the country with numerically the most experience in orchestrating phase 2 and 3 clinical trials—a total of 635 trials compared with the UK at 522 trials. This analysis cannot intimate reasons for Germany’s excellence in clinical trial implementation but given that the ethical frameworks for Germany and the UK are comparable, possible explanations include a large haemato-oncology patient population as well as good trial infrastructure leading to fast start up of clinical trials and high post-trial market potential. Similarly, the regulatory environment in Germany via the Arzneimittelmarkt-Neuordnungsgesetz (Pharmaceuticals Market Reorganisation Act) procedure encourages collaboration on research to help support the pricing of drugs.
This analysis demonstrates that in the UK, clinical trials when started are more likely to be completed and when completed translate into highly robust publications and patent applications. In phase 1 studies, the UK is the leader across the board, and in haematological cancers only second to France. Given the excellence in early phase clinical trials in the UK, there is a need to explore how improvements in later phase 2 and 3 clinical trials can be achieved. Increasing the number of phase 2 and 3 trials undertaken that can be completed and translated into strong citable communicable data will set the UK apart as a place for researchers, academic trial funding and pharma opportunity. Phase 3 clinical trials are the point where patients can directly access and benefit from medications for their cancer. Options that might be considered to improve the proportion of phase 3 studies undertaken in the UK include shortening of regulatory time frames and financial competitive advantages. The UK was shown to be the slowest at 186 days for the regulatory process, which will impact the consideration of the UK as a country of interest. Optimising this time frame needs to be considered, likely at a governmental level, to bring approvals in line with other countries such as Germany and the USA, particularly in a post-Brexit era where access to the centralised approvals system through the European Medicines Agency is no longer available. Similarly, there is a need to streamline CTA and ethics procedures. This process is underway through the collaboration of the MHRA with the National Institute for Health Research (NIHR) to combine procedures of Clinical Trials of Investigational Medical Products and Clinical Trial Authorisation and Research Ethics Committee opinion. This will comprise the largest overhaul of trial regulation for several decades and accompanies a UK Government commitment to increase clinical trial investment from approximately £9 billion in 2017 (the timing of this analysis) to £22 billion by 2026–2027. Such a combined way of working may improve approval times. Delays in trial startups are linked to substantial study delays and costs which can threaten the feasibility of a trial, delay access to treatment for patients and lead to lost opportunity costs.21 22 But, this may require digital transformation and support of digital capabilities, as it is clear that biopharma excellence and digital excellence may not be tracked together.23 With a median trial set up time for the UK of 6 months, double that of Germany, success of this initiative is a priority. There is a case for central incentivisation of clinical trials in the UK, as happens in the USA, that is strategically applied to centres of excellence providing innovation.24 The joint MHRA and NIHR approach may already be providing benefits with a reduction in clinical trial start up times already reported. Embracing the increasing patient centricity of clinical trials through use of the NHS may provide the UK with a competitive advantage in clinical trial design and recruitment without compromising on time to trial implementation.
Reinvigorating the clinical trial landscape in the UK—particularly following Brexit, the pandemic and an economic downturn—is essential for the fiscal benefits it brings and the benefits to patients in accessing innovative medications. The NHS as a national structure provides multiple centres of excellence and Clinical Research Networks that are highly respected worldwide. However, the NHS continues to operate under increasing burdens, including insufficient funding, patient waiting lists, staff shortages, an ageing population and evolving healthcare needs. As such, the system remains at capacity with the capability to undertake research seriously compromised.
However, both the UK and Europe lag behind the USA in terms of clinical trial investment and countries such as China are increasingly emerging as attractive locations for studies.25 A connected, less siloed, approach to clinical trial implementation within the UK alone and within the broader context of Europe is required.
Limitations
There are several limitations to these data. From observed data, it has been inferred that the UK may be a less attractive country for clinical trials than others in Europe, however, nuances to this observation have not been evaluated. It may be that a subjective analysis of pharmaceutical companies and clinical research organisations, perhaps by survey, might provide a different or deeper insight into why the UK up to 2017 was seemingly a more difficult country to navigate for late-phase clinical studies. The outcomes we observed suggested that perhaps regulatory hurdles were proving to be barriers to later-phase clinical trials, but the impact of a centralised approval structure in the UK has not been evaluated. It may be that current constraints faced by the NHS may, in part, render clinical development in the UK less attractive. This would be an interesting topic for further study. It is possible that limitations of infrastructure may be a contributing factor to the high number of sites recruited for clinical studies which never recruit patients.
The data in this study provide insight into haematological cancer clinical trials up to and including 2017. The data cut-off of 2017 is a challenge; however, these data do allow us to fully understand the UK’s capability without the significant uncertainty of the Brexit vote and subsequent COVID-19 pandemic. It would be useful to explore the impact on the UK on both of these events since this research. While the data obtained reflects the situation in the UK up to, and including 2017, the situation in the UK, post-Brexit and postpandemic cannot be known. Given the lifecycle of clinical trials is approximately 10 years, it would be interesting to explore the UK’s position with respect to clinical trials in 2027 or beyond. At this time point, the landscape should reflect a normalcy following Brexit, be relieved of any masking of effect by the COVID-19 pandemic and reflect more clearly the government initiatives currently in place, which are beginning to provide benefits which we hope will be continued by the new UK government. Other limitations not explored in this analysis include cost of developing and implementing clinical trials in the UK and other countries and the potential for governmental incentives to improve attractiveness. Understanding the role of geographical location for clinical trials requires an analysis of willingness of countries to invest in clinical studies, their landscape of current healthcare funding, clinical trial capacity from the perspective of the potential patient population and the willingness of patients to be involved in research. These would all be of interest for future study.
Conclusions
Based on 2017 data, the UK lags behind other European countries as a hub for clinical trial development and implementation. The UK, however, when it does undertake clinical trials, clearly provides data that are meaningful and relevant and ultimately useful to drug development and future patient care. As the post-Brexit, postpandemic landscape shifts in the UK, it may be that future data will show an increase in the volume and impact of clinical development in the UK. Since the pandemic, the UK government proposed to improve the UK as a clinical trials hub. Its response has been to explore the role of telemedicine in trials, the definition of a ‘trial’ site, and to revise ways of working in line with recommendations from the Taskforce for Innovation, Growth and Regulatory Reform including ICH E6 guidance on good clinical practice and the development of an Innovative Licensing and Access Pathway. In this way, effective and efficient pathways to bring innovative medicines to patients earlier should be possible with the UK as a hub for clinical trials.23
Data availability statement
Data are available on reasonable request. All data are available on reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
Not required.
Acknowledgments
Writing assistance and editorial support were provided by Susan Allen, Brandfish Limited UK and funded by Amryt Pharmaceuticals. The analysis was conducted as part of a successfully defended and awarded MSc thesis from King’s College London, by MA funded by Celgene and Amryt Pharmaceuticals.
Footnotes
Contributors MA is the guarantor. MA was responsible for developing and critiquing the survey, data collation and data preparation, data analysis and interpretation, developing the concept and outline of the manuscript and writing, reviewing and revising the manuscript. JB was responsible for critiquing the survey, data interpretation, developing writing, reviewing and revising the manuscript. AeB was responsible for critiquing the survey, data interpretation, developing writing, reviewing and revising the manuscript. AK and JB were responsible for critiquing the survey, data interpretation, developing writing, reviewing and revising the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests The authors declare there are no current conflicts of interest. MA, JB and AK were employees of Celgene at the time the analysis was conducted. The analysis was conducted as part of research for a successfully defended MSc thesis for MA, which was funded by Celgene and Amryt Pharmaceuticals.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.