Article Text
Abstract
Objective To assess the pooled in-hospital mortality among neonates with necrotising enterocolitis (NEC) in Ethiopia.
Design This was a systematic review and meta-analysis reported based on the Preferred Reporting Items for Systematic Review and Meta-analysis guideline.
Data sources African Journals Online, PubMed/Medline, Google Scholar, Cochrane Library and repositories of Ethiopian Universities.
Eligibility criteria Published and unpublished articles that had reported the in-hospital mortality among neonates with NEC in Ethiopia were included, whereas, articles with no abstracts and/or inaccessible full texts, citations, reviews, commentaries editorials, conference abstracts, anonymous reports and articles reported in non-English language were excluded.
Data extraction and synthesis Articles that passed the eligibility criteria were assessed for their quality using the quality appraisal criteria for prevalence studies. Data extraction and cleaning were done by using the Microsoft Excel work sheet, and data were analysed by STATA V.11.0 using the random effects model at 95% CI. Test of heterogeneity, publication bias, sensitivity analysis, subgroup analysis and meta-regression were performed.
Results A total of 12 articles involving 588 neonates were included. The pooled in-hospital mortality among neonates with NEC in Ethiopia was found to be 70.0% (95% CI=60.0% to 80.0%; I2=87.5%). There was significant difference in mortality by study population as the in-hospital mortality among neonates with NEC was 83.0% (95% CI=76.0% to 89.0%; I2=42.8%; five studies) in preterm neonates and 73.0% (95% CI=60.0% to 86.0%; I2=66.3%; four studies) in low birthweight neonates (p<0.001).
Conclusion The in-hospital mortality of neonates with NEC in Ethiopia was found to be high in which 7 out of 10 neonates diagnosed with NEC ends with death. Therefore, the currently available NEC prevention strategies should be evaluated for individual units and introduced where possible.
- Mortality
- NEONATOLOGY
- INTENSIVE & CRITICAL CARE
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Not applicable.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The major strength of this study is that it is the first systematic review and meta-analysis of in-hospital mortality among neonates with necrotising enterocolitis (NEC) in Ethiopia.
Almost all of the included studies did not clearly state their criteria for the diagnosis of NEC, apart from saying physician diagnosis was used.
The observed heterogeneity among the included studies was high.
Introduction
Necrotising enterocolitis (NEC) is an inflammatory disorder characterised by inflammation of the gastrointestinal tract, bacterial invasion, cellular damage and ischaemic necrosis of the intestines.1 Bell’s criteria define three stages of NEC.2 The first stage (stage I) is characterised by a broad spectrum of non-specific symptoms and/or signs like poor feeding, feeding intolerance or vomiting and mild abdominal distension, which help the clinician to suspect NEC. The second stage (stage II) represents NEC that has been confirmed with abdominal radiographs (definite NEC), and the last stage (stage III) represents an advanced NEC characterised by complications including intestinal perforation, septic shock, disseminated intravascular coagulation, neutropenia, ascites and other life-threatening conditions.1–3
NEC is a disorder of neonates, and neonates with prematurity, low birth weight (LBW) and cardiopulmonary abnormalities (severe respiratory distress, perinatal asphyxia and congenital heart diseases) have an increased risk of acquiring NEC.4–8 Formula feeding, aggressive enteral feeding and umbilical catheterisation have also been identified as risk factors for the disease.8–10 More than 70% of NEC cases occur in preterm neonates, and 10%–15% of cases occur in term neonates.11 12 It affects 2%–13% of preterm and LBW neonates, and it is a cause for nearly 8% of all neonatal intensive care unit (NICU) admissions.1 The global incidence of NEC in neonates has been estimated to reach as high as 7.0%. Due to the advancements of neonatal care and improvements of survival among preterm neonates, the global burden of NEC has been increased over time.13 Low-income countries were found to have lower prevalence of NEC than high-income countries. But, in Ethiopia, the prevalence of NEC ranges from 2.1% to 25.4% among NICU admitted neonates.13–15
NEC is a devastating disease with a mortality rate of 10%–50%. However, mortality might approach 100% among neonates with advanced stages of NEC.1 In Ethiopia, a number of studies have reported the in-hospital mortality of neonates diagnosed with NEC. But reported results were highly variable, ranging from 44.9% to 89.3%.15 16 Despite this, pooled prevalence has not been reported to date. Therefore, this systematic review and meta-analysis was aimed to assess the pooled in-hospital mortality among neonates with NEC in Ethiopia.
Methods
Reporting guideline
The Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) was used as a guide for organising and reporting this systematic review and meta-analysis.17
Databases and search strategy
For this systematic review and meta-analysis, African Journals Online, PubMed/Medline, Google Scholar, Cochrane Library and repositories of Ethiopian Universities were the electronic databases that were used as a source of information. Additionally, probing for grey literature and snow ball searching were executed to address potentially relevant articles. Searching was conducted from 15 to 25 December 2023. To have an inclusive search string, the main terms used for searching were ‘Necrotizing Enterocolitis’ and ‘Ethiopia’. The Boolean operator ‘AND’ was used to connect those terms accordingly. No filters were applied. The detail of search string used for each database has been presented in online supplemental table 1.
Supplemental material
Eligibility criteria, quality assessment and study selection process
In this systematic review and meta-analysis, articles were selected in accordance with the following predetermined inclusion and exclusion criteria. Published and unpublished articles that had reported the magnitude of mortality among neonates with NEC in Ethiopia were included. On the other hand, articles with no abstracts and/or inaccessible full texts, citations, reviews, commentaries, editorials, conference abstracts, anonymous reports and articles reported in non-English language were excluded. Additionally, for articles that had used the same data set, one of the articles with precise results (larger sample size) was included and the others were excluded. After retrieving, duplicates were removed using the EndNote reference managing software V.7.0, and then two authors (MB and MA) reviewed the remaining articles against the predetermined eligibility criteria by their title and/or abstract. Articles that passed the eligibility criteria were assessed for their quality by another two authors (FG and AG). The quality of included studies was assessed by using the Hoy et al risk of bias assessment tool for prevalence studies.18 19 Disagreements between appraisers were solved by incorporating third person (AW), and articles with a score ≤3 (studies having low risk of bias) were included for analysis (online supplemental table 2). The full flow diagram of the study selection process has been depicted in figure 1.
Supplemental material
Preferred Reporting Items for Systematic Review and Meta-analysis flow diagram showing the identification, screening, reasons for exclusion and number of included research articles in this systematic review and meta-analysis; 2024.
Outcome variable
The outcome variable of this systematic review and meta-analysis was in-hospital mortality of neonates with NEC in Ethiopia.
Patient or public involvement
No direct patient or public involvement.
Data extraction process, data synthesis and presentation
Data extraction and cleaning was done by using Microsoft Excel worksheets. After this, data were exported to STATA V.11.0 for analysis. SEs of the prevalence were calculated according to the assumption of binomial distribution formula as: SE of prevalence = 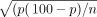 ; where
p
is the prevalence and
n
is the sample size. Pooling of the outcome variable (prevalence of in-hospital mortality among neonates with NEC) was first tested by the classic ‘Metan’ command with weighted inverse variance random effects model at 95% CI, but some study specific estimates were found to have inadmissible CIs (>100%). Therefore, pooling was done based on the ‘Metaprop’ command with the random effects model at 95% CI. The ‘Metaprop’ command is an adaptation of the classic ‘Metan’ command. It is considered as the preferred pooling method for binomially distributed data with smaller sample sizes, estimates near to or on the margin (0% or 100%) or larger SEs; especially if the classic ‘Metan’ command yields inadmissible CIs (for percentage, if it results in a CI of >100% or <0%). The ‘Metaprop’ command computes the 95% CI using the score statistic and the exact binomial method by incorporating the Freeman-Tukey double arcsine transformation of estimates (proportions/prevalence), yielding admissible CIs (0% to 100%).20
; where
p
is the prevalence and
n
is the sample size. Pooling of the outcome variable (prevalence of in-hospital mortality among neonates with NEC) was first tested by the classic ‘Metan’ command with weighted inverse variance random effects model at 95% CI, but some study specific estimates were found to have inadmissible CIs (>100%). Therefore, pooling was done based on the ‘Metaprop’ command with the random effects model at 95% CI. The ‘Metaprop’ command is an adaptation of the classic ‘Metan’ command. It is considered as the preferred pooling method for binomially distributed data with smaller sample sizes, estimates near to or on the margin (0% or 100%) or larger SEs; especially if the classic ‘Metan’ command yields inadmissible CIs (for percentage, if it results in a CI of >100% or <0%). The ‘Metaprop’ command computes the 95% CI using the score statistic and the exact binomial method by incorporating the Freeman-Tukey double arcsine transformation of estimates (proportions/prevalence), yielding admissible CIs (0% to 100%).20
Heterogeneity among the included studies was assessed by the Higgin’s I2 with its corresponding p value, and I2≥50% and/ or p value<0.05 was used to declare significant heterogeneity. Presence of publication bias was also evaluated by the funnel plot and Egger’s regression test, and asymmetrical distribution on funnel plots and non-significant Egger’s regression test (Bias≥0.05) was used to assert the absence of publication bias. A leave-one-out sensitivity analysis was also employed to assess if there are influential studies that had greatly affected the pooled estimate. Subgroup analysis and meta-regression were used to identify possible sources of heterogeneity accordingly.
Results
Study selection and characteristics of the included articles
From the electronic database search, a total of 1948 articles were retrieved for screening. The whole process of study selection has been illustrated as a PRISMA flow diagram (figure 1). A total of 12 studies14–16 21–29 were included in the final analysis. Most of the studies were conducted among preterm and/or LBW neonates. Seven of the included articles were cohort studies, while the remaining five articles were cross-sectional studies. The included articles were published between 2016 and 2022. Five of the included studies were conducted in Amhara region, four in Addis Ababa, two in Southern Nations, Nationalities, and Peoples’ region and one in Tigray region. The full characteristics of the included studies have been presented in tables (online supplemental table 3).
Supplemental material
In-hospital mortality among neonates with NEC
Among the included studies, the lowest in-hospital mortality among neonates with NEC in Ethiopia was 44.9%, while the highest was 89.3%. Overall, the pooled in-hospital mortality among neonates with NEC in Ethiopia was found to be 70.0% (95% CI=60.0% to 80.0%; I2=87.5%; p<0.001; 12 studies) (figure 2).
Forest plot showing the proportion of in-hospital mortality among neonates with necrotising enterocolitis (NEC) in Ethiopia, 2024.
Heterogeneity, publication bias and sensitivity analysis
In this meta-analysis, there was a high heterogeneity among the included studies as evidenced by I2=87.5% and significant p value (<0.001). On the other hand, the funnel plot showed symmetrical distribution (figure 3) and the Egger’s test was non-significant (p value=0.218), indicating the reduced risk of publication bias in this meta-analysis. Results from the leave-one-out sensitivity analysis had also showed all estimates to be between 68.0% and 73.0% and estimates were within the 95% CI limits of the pooled estimate (60.0% to 80.0%), assuring the absence of an influential study that potentially affected the exhibited pooled result (online supplemental table 4).
Supplemental material
Funnel plot showing the symmetrical distribution of included studies, 2024. NEC, necrotising enterocolitis.
Subgroup analysis
Subgroup analysis was performed by study design, study region, study population and publication year. Regarding the study design, higher in-hospital mortality among neonates with NEC in Ethiopia was reported among studies conducted with cohort design (pooled prevalence=75.0%; 95% CI=67.0% to 82.0%; I2=37.5%; p=0.14; seven studies). The prevalence was 63.0% (95% CI=44.0% to 83.0%; I2=94.9%; p<0.001; five studies) among studies conducted with cross-sectional design. But, this difference was not statistically significant (p value for heterogeneity between groups<0.001). There was significant difference in mortality by study population as the in-hospital mortality of neonates with NEC was 83.0% (95% CI=76.0% to 89.0%; I2=42.8%; p=0.14; five studies) among preterm neonates and 73.0% (95% CI=60.0% to 86.0%; I2=66.3%; p=0.03; four studies) among LBW neonates (p value for heterogeneity between groups<0.001). There was no statistically significant difference in mortality by study region as the in-hospital mortality was 72.0% (95% CI=60.0% to 85.0%; I2=75.8%; p<0.001; five studies) in Amhara region, 70.0% (95% CI=50.0% to 90.0%; I2=94.1%; p<0.001; four studies) in Addis Ababa and 57.0% (95% CI=46.0% to 67.0%; two studies) in Southern Ethiopia (p value for heterogeneity between groups=0.199). Regarding the study year, mortality was 69.0% (95% CI=50.0% to 88.0%) and 71.0% (95% CI=58.0% to 84.0%) among studies conducted before 2022 and among studies conducted from 2022 to 2023 (p value for heterogeneity between groups=0.878).
Meta-regression
A preplanned meta-regression was also conducted to evaluate if sample size, study design, study year, study population and study region have an effect on variability of the in-hospital mortality among neonates with NEC in Ethiopia and to test if those variables were the possible causes for the observed high heterogeneity among included studies. Hence, sample size, study design, study year and study region were not found to be a statistically significant cause of heterogeneity and variability (p value>0.05). On the other hand, study population (grouped as preterm, LBW, both preterm and LBW, PNA and all neonates) was found to have an effect on the variability of in-hospital mortality among neonates with NEC and was a possible source of heterogeneity among the included studies (p value=0.004; adjusted R2=70.3%; residual I2=60.9%).
Discussion
This study was aimed to determine the in-hospital mortality among neonates with NEC in Ethiopia. A total of 12 articles involving 588 neonates having NEC were included in this analysis. Of the 588 neonates with NEC, 408 died. In this systematic review and meta-analysis, the ‘Metaprop’ command with 95% CI was employed. Among the included studies, the lowest in-hospital mortality among neonates with NEC in Ethiopia was 44.9%, while the highest was 89.3%. There was statistically significant heterogeneity among the included articles. The overall in-hospital mortality among neonates with NEC in Ethiopia was found to be 70.0% (95% CI=60.0% to 80.0%; I2=87.5%; p<0.001).
The findings of this meta-analysis of NEC mortality were higher than studies conducted in South Africa (39.9%),30 Poland (21.0%)31, Italy (44.5%),32 China (29.8%),33 Saudi Arabia (37.0%),34 the USA (26.5%)35 and Southwest China (19.6%).36 This discrepancy might be due to differences in socioeconomic status, characteristics of the study participants and differences in perinatal and neonatal care between Ethiopia and those countries. A systematic review, conducted by Jones and Hall, revealed the overall mortality of neonates with NEC to be 23.5%.37 All of the countries of that review were high-income countries, and differences in income level might explain the difference between that review and this meta-analysis. A study from Zambia had reported that the mortality among neonates with NEC to be 77.8%38, which was nearly similar to the findings of this meta-analysis. This congruency might be due to nearly similar socioeconomic status between Zambia and Ethiopia. A study from Indonesia had also reported that the mortality of neonates with NEC to be 72.7%39, but that study was only on preterm neonates.
NEC has a significant mortality rate worldwide. Especially, in developing countries like Ethiopia, NEC mortality is high. Probiotic supplementation has been identified to have an effect on the occurrence and associated mortality of NEC. A recent meta-analysis had revealed that probiotics can significantly reduce the incidence of NEC (RR=0.436; 95% CI=0.357 to 0.531; p value<0.001) and NEC related mortality (RR=0.639; 95% CI=0.423 to 0.966; p value=0.034) in neonates.40 However, there are still unanswered questions about the quality, safety, dosage and duration of probiotic supplementation. Human milk feeding has also been identified as an effective approach for reducing the incidence of NEC by a number of clinical trials. Initial empiric antibiotic administration in neonates was found to increase the risk of NEC. Antenatal steroids, standardised feeding protocols, early initiation and advancement of enteral feeding with human milk, non-nutritive sucking, strict infection prevention measures and early diagnosis and management of NEC were also identified as strategies for preventing NEC and reducing NEC associated mortality.41–44 Therefore, those strategies might be helpful to lower the exhibited high burden of mortality among neonates with NEC.
In this systematic review and meta-analysis, there was statistically significant difference in mortality between preterm and LBW neonates having NEC. The in-hospital mortality of neonates with NEC was 83.0% (95% CI=76.0% to 89.0%) among preterm neonates and 73.0% (95% CI=60.0% to 86.0%) among LBW neonates. Studies have identified prematurity to be the leading factor for NEC and preterm neonates had higher risk of developing NEC than LBW neonates.8 LBW might be due to prematurity or small for gestational age.45 This implies that term neonates might have LBW and term neonates had lower risk of developing NEC and dying from NEC.46 47 Hence, as supported by this meta-analysis, preterm neonates might have higher risks for mortality due to NEC than LBW neonates. The meta-regression result of this analysis had also showed study population difference to be a significant cause of high heterogeneity exhibited among the included studies. The adjusted R2 was 70.3%, implying that difference in study population could explain 70.3% of the exhibited heterogeneity.
This study is the first systematic review and meta-analysis of in-hospital mortality among neonates with NEC in Ethiopia. Pooling was done by using the ‘Metaprop’ command which is considered as the most appropriate method of pooling binomially distributed data such as proportions and prevalence. However, this systematic review and meta-analysis might not be free from limitations. First, the observed heterogeneity among the included studies was high. But, sensitivity analysis, subgroup analysis and meta-regression were performed accordingly to investigate the source of heterogeneity. Assessment of publication bias was also employed. Second, as a limited number of studies were included, the results of this study might not indicate the nation-wide estimate. At last, almost all of the included studies did not state their criteria for the diagnosis of NEC. They simply used physician diagnosis and did not define how the physician diagnosis was made.
Conclusion and recommendations
Despite the advancements of neonatal care and a slight reduction of neonatal mortality in the last decades, the in-hospital mortality of neonates with NEC in Ethiopia was found to be high. As per this meta-analysis, 7 out of 10 neonates diagnosed with NEC ends with death in Ethiopia. Studies had also revealed the prevalence of NEC among neonates in Ethiopia to be as high as 25.4%. Therefore, NEC preventive mechanisms like human milk feeding, antenatal steroids, standardised feeding protocols, early initiation and advancement of enteral feeding with human milk, non-nutritive sucking, strict infection prevention measures, early diagnosis and management of NEC and cautious use or cessation of initial empiric antibiotic administration in neonates might be helpful to decrease the incidence and in-hospital mortality of neonates with NEC. These currently available NEC prevention strategies should be evaluated for individual units and introduced where possible.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Not applicable.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
We would like to acknowledge authors of the included articles.
References
Footnotes
Contributors All authors of this article had made substantial contributions to the conception of this study. MB and MA assessed articles based on the predetermined criteria; AG and FG assessed the quality of eligible articles; and AW had involved in the quality assessment to solve disagreements between the two (AG and FG) assessors. MB, MA and ND involved in the extraction, analysis and interpretation of data; all authors took part in drafting and revision of the article; agreed to submit to the current journal; approved the final version to be published; and agree to be accountable for all aspects of the article. MB is responsible for the overall content (as guarantor).
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.





