Article Text
Abstract
Objectives Centres of clinical excellence (CoCE) are healthcare facilities that provide excellent healthcare. However, despite their increasing prevalence, it is unclear how CoCE are identified and monitored. This paper explores how CoCE has been described in the literature, including its defining characteristics and selection and monitoring processes.
Design We conducted a scoping review following Arksey and O’Malley’s framework, enhanced by Levac et al. Additionally, we adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews guidelines.
Data sources A comprehensive search using MEDLINE Ovid, PubMed, Web of Science, CINAHL and Scopus was conducted to identify relevant literature from January 2010 to June 2022.
Eligibility criteria for selecting studies We included published studies and grey literature that described how a CoCE was defined, established, monitored or evaluated.
Data extraction and synthesis Two independent reviewers completed the title and abstract screening, reviewed the full texts and extracted data.
Results 50 records describing 45 initiatives were included. More than half were published in the USA (n=25, 56%). All but one initiative focused on one clinical condition/population, most commonly cardiovascular disease (n=8, 17%), spinal surgeries (n=4, 9%) and pituitary tumours (n=4, 9%). Most initiatives (n=30, 67%) described a structured process to establish CoCE. The definitions of CoCE were not uniform. Common defining features included the volume of patients treated, medical expertise, a highly skilled multidisciplinary team, high-quality care and excellent patient outcomes. Identification as a CoCE varied from self-identification with no explicit criteria to application and assessment by an approval panel.
Conclusion Despite a growing prevalence of CoCE, there are inconsistencies in how CoCE are established, identified, monitored and evaluated. Common (but not uniform) features of CoCE are highly skilled staff, high-quality care delivery and optimal patient outcomes.
- Organisation of health services
- Protocols & guidelines
- Quality in health care
- International health services
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Organisation of health services
- Protocols & guidelines
- Quality in health care
- International health services
STRENGTHS AND LIMITATIONS OF THIS STUDY
The study used inclusive search strategies (peer-reviewed journals and grey literature) and a stringent review process using two independent reviewers throughout the process.
The study used Arksey and O’Malley’s framework with enhancement from Levac et al and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews guidelines.
We may have missed established centres of clinical excellence that have not published any studies or reports or published in non-indexed sources.
Introduction
Healthcare facilities worldwide have a shared goal to continually improve healthcare delivery, often using stringent standards and indicators.1 2 Improvements in healthcare delivery can take the form of defining best clinical practice or demonstrating important aspects of care, such as safety, access, affordability, equity, effectiveness and efficiency.
Most healthcare organisations must meet national quality and safety standards to address clinical practice and organisational performance.1 Accreditation is instrumental in achieving a baseline standard of care; however, it is not usually designed to recognise excellent care or to optimise patient-reported outcomes and experience. Recognising this gap between care that meets accreditation standards and ‘excellent’ care, some healthcare facilities are taking proactive steps to engage in self-improvement and seek recognition for delivering exceptional care.
Excellence within healthcare is often labelled ‘clinical excellence’,3 and organisations that deliver exceptional patient care have been called centres of excellence or centres of clinical excellence (CoCE).4–6 Other dimensions of excellence that have been described in healthcare include ‘research excellence’,7 ‘service excellence’8 and ‘operational excellence’.9 A recently published review6 summarised evidence pertaining to centres of excellence in healthcare, education, research, industry and information technology. The authors of this review concluded that there are inconsistencies in how healthcare facilities are designated as centres of excellence and ambiguity between centres of excellence and regular healthcare facilities, with limited information on how these centres were evaluated. Similarly, research excellence has been reviewed from education and clinical research perspectives, and frameworks are frequently not comprehensive,6 with unclear methods used to determine excellence.
Attaining recognition as a CoCE could be a source of inspiration to facilities that are recognised as leads in healthcare provision.10 Health professionals within the facilities can be inspired to pursue and maintain the best clinical care for their patients by promoting high-quality, up-to-date, evidence-based care to their community.10 Additionally, CoCE can work with accreditation bodies to set higher benchmarks that encourage innovative patient-centred care. Accreditation bodies can adopt and maintain advanced standards of care over time, helping healthcare centres to continually raise the standards of patient outcomes.11
Despite the increasing use of the term CoCE, there is a lack of clarity about how this term is defined, how sites are nominated and selected as CoCE and how CoCE are evaluated and monitored. Therefore, the primary aim of this scoping review was to map evidence on CoCE in healthcare. We sought to explore and answer the following questions systematically:
What CoCE have been described in the literature?
What are the defining characteristics of CoCE?
How are CoCE selected or nominated?
What monitoring processes are employed to remain as CoCE?
Through conducting this review, we planned to explore the multifaceted dimensions of CoCE.
Method
Protocol and registration
We registered the scoping review protocol on Open Science Framework. We employed the scoping review framework proposed by Arksey and O’Malley12 with the refinement outlined by Levac et al 13 to evaluate the evidence on CoCE. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews.14
Identifying relevant studies
We developed a search strategy with the support of a research librarian (online supplemental file 1). We searched MEDLINE Ovid, PubMed, Web of Science, CINAHL and Scopus to identify published records between January 2010 and June 2022. We also searched for grey literature (government reports, policies, protocols, conference proceedings and unpublished studies) and relevant websites using Google and Google Scholar. We also searched the reference lists of included records to check for further relevant records.
Supplemental material
Inclusion and exclusion criteria are presented in box 1. We included records that discussed CoCE that provided clinical care for people with any health condition in any setting (primary care, inpatient, outpatient or community). To be included, records had to describe how a CoCE was defined, established, monitored or evaluated. We excluded records that used the term ‘CoCE’ without outlining any criteria. Centres of excellence that were not designed to provide clinical care (such as centres of research excellence) were excluded. Given the exploratory nature of the research questions, there was no limitation to study populations or interventions.
Eligibility criteria for article selection
Inclusion criteria
Available in the English language.
Information on CoCE.
Healthcare organisations or services providing clinical care to people with any healthcare condition.
Published from January 2010.
Any geographical location.
Studies describing the development/defining/monitoring/evaluation/frameworks of CoCE.
Exclusion criteria
Records that describe a study conducted at CoCE (eg, using participants from CoCE).
Centres that do not provide clinical care (eg, Centres of Research Excellence or Centres of Leadership Excellence).
Conference abstracts/papers, letters, NICE guidelines, JBI guidelines.
Only looking at costs associated with one CoCE (no comparator).
Only looking at clinical outcomes for people receiving care at CoCE (no comparator).
Using the term ‘CoCE’ without outlining the criteria.
CoCE, centres of clinical excellence; JBI, Joanna Briggs Institute; NICE, National Institute for Health and Care Excellence.
Study selection
The search results were imported into Covidence, and duplicates were removed. As recommended by Levac et al,13 two reviewers independently screened titles and abstracts and reviewed full-text documents using the inclusion criteria (see box 1). One reviewer (TK) conducted the online search for relevant websites (first 20 pages on Google search) and two reviewers (TK and LNB) independently completed the screening and review of the grey literature. The inclusion and exclusion criteria were reviewed periodically throughout the title and abstract screening process to ensure the criteria facilitated the identification and inclusion of relevant studies.
Charting the data
A data extraction form was developed for the study (online supplemental tables 1 and 2). We pilot-tested the extraction form with the first 15 eligible records to ensure consistent data collection. Two reviewers (TK and EAL) independently extracted data on all included studies using the extraction form on Covidence. The quality of individual records was not assessed due to the descriptive nature of the review aims.
Supplemental material
Supplemental material
Collating, summarising and reporting the results
We synthesised the research findings according to the research questions and presented data from all included studies in tables. Study characteristics were presented descriptively, and the research questions were presented narratively. Henceforth, the CoCE will be identified as initiatives and the search results will be defined as records. Each initiative will be described either as a theoretical centre (describing aspirational criteria/frameworks to develop a CoCE) or a physical centre where clinical care is provided. Initiatives that described a framework were classified as ‘creating’ a framework, ‘using’ or ‘adapting’ a pre-existing framework.
Patient and public involvement
Patients were not involved in the design or completion of this study.
Results
Selection of sources of evidence
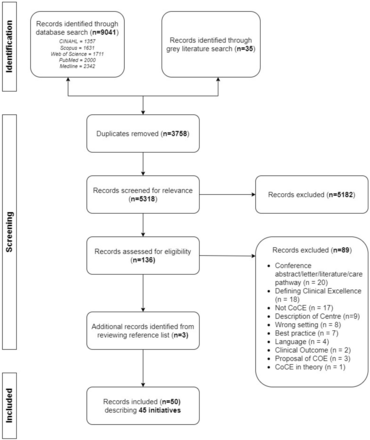
Overall, 9077 records were identified from a database search, and 36 records were identified through a grey literature search. A further three records were identified by reviewing reference lists of included records. 50 records describing 45 CoCE initiatives were included in the analysis (figure 1). The complete search results and strategies are available in online supplemental file 1. Among the records excluded at full-text review, 25 (n=28%) records described or labelled a centre as a CoCE but did not provide any selection criteria or any details about how the centres were nominated or monitored.
PRISMA flow diagram. CoCE, centres of clinical excellence; COE, centre of excellence; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of sources of evidence
Most records (n=43, 86%) were published in or after 2015. Nearly all the included records (n=44, 88%) were published in peer-reviewed journals, but only 15 (30%) were research articles, the remaining 28 (56%) records were other article types such as editorials or case reports. Two websites were identified as additional records for initiatives identified through the literature search (see tables 1 and 2).
Characteristics of included records
Characteristics of CoCE initiatives
Synthesis of results
Less than half (n=20, 47%) of the identified initiatives were physical CoCE. With the exception of one CoCE which provided care for people with diabetes and cardiovascular disease,15 all identified CoCE treated a single clinical condition or population. The most commonly described conditions were cardiovascular disease16–23 (n=8, 17%), spinal surgeries24–27 (n=4, 9%), pituitary tumours28–31 (n=4, 9%), diabetes15 32 33 (n=3, 6%) and obstetrics34 35 (n=2, 4%).
Some CoCE (n=6, 13%) were located across several countries,17 21 25 28 36 37 whereas the majority were described as stand-alone clinical centres, such as wards, surgical centres or clinics. Eight CoCE (18%) were located in low-income and middle-income countries.20 32 38–43 More than half of the included CoCE were located in the USA (n=25, 53%). CoCE established in high-income countries were typically described in terms of high quality of care delivery, such as standardised care and optimal outcome (n=12, 27%),19 21–23 25 33 35 38 39 44–46 comprehensive multidisciplinary care (n=8, 18%)16 28 31 32 40 47–49 or accessible patient-centred care (n=7, 16%).4 15 29 36 42 50–52
More than half of the initiatives (n=30, 67%) described a structured process to establish a CoCE. While many initiatives reported that the CoCE was established using a framework or series of developmental stages, details regarding the developmental stages were rarely available. Five initiatives were reported using published frameworks (Elrod and Fortenberry,29 37 45 Christmas53 and National Cancer Institute23) to guide their process to establish the CoCE (see table 2 for further details).
Defining characteristics of CoCE
Less than half (n=19, 42%) of the initiatives explicitly defined the characteristics of the CoCE. Seven (16%) initiatives20 24 26 29 37 45 54 used the definition from Elrod and Fortenberry4: ‘a programme within a healthcare institution which is assembled to supply an exceptionally high concentration of expertise and related resource centred on a particular area of medicine, delivering associated care in a comprehensive, interdisciplinary fashion to afford the best patient outcomes possible’ (p.16).4
High volumes of patients treated or numbers of procedures performed, staffing, infrastructure, high quality of care and above-average patient outcomes were the most commonly described defining features of CoCE. Staffing components included medical expertise, highly skilled multidisciplinary teams and staff-to-patient ratios. Other resources that were described as part of the CoCEs were infrastructure (n=15, 33%), such as building space and examination rooms and specialised equipment (n=9, 20%). High quality of care delivery was described in terms of standardised care and optimal outcome (n=12, 27%),19 21–23 25 33 35 38 39 44–46 comprehensive multidisciplinary care (n=8, 18%)16 28 31 32 40 47–49 or accessible patient-centred care (n=7, 16%).4 15 29 36 42 50 51 The availability of treatment protocols was described as an important feature in seven initiatives (15%) (see table 2 for details).
There were differences noted in the defining characteristics of CoCE in low-income, middle-income and high-income countries. Universally, most CoCE had common features regarding staff expertise, equipment and patient outcomes. However, CoCE in low-income and middle-income countries tended to provide a healthcare service that otherwise was not available in the region, for instance, neurosurgery in Peru39 and comprehensive dental care in Guwahati, India.38
Selection or nomination process of CoCE
No details were available about how sites were selected as CoCE in half (n=24, 53%) of the included initiatives. While 21 initiatives reported that there was a selection or nomination process to be recognised as a CoCE, the details of the selection or nomination process were inconsistently reported. When reported, processes used to select centres as CoCE were varied and included application and assessment by an approval panel (n=9, 45%),4 8 23 34 43 45 54–56 self-identification as a CoCE with no explicit criteria or external assessment (n=6, 30%)15 16 19 29 41 50 and site visit by funding body to assess suitability (n=1, 5%).18 Only four (20%)36 46 48 57 initiatives presented the process used to select the CoCE in its entirety, which are presented in table 3. The bodies providing oversight of the nomination or selection of the CoCE were professional bodies,23 26 34 36 48 54 insurers45 55 and organisations.4 38 46
Outline of selection/nomination process of CoCE
Monitoring protocols to remain a designated centre of clinical excellence
Only 24 (53%) of the included initiatives reported a monitoring process for the CoCE. Monitoring was mandatory for 6 (25%)34 36 48 52 54 57 initiatives through recertification process. Other initiatives reported the importance of monitoring outcomes such as productivity (n=5, 21%),17 19 39 43 50 patient outcomes (n=9, 36%),15 16 27 29 30 32 45 47 49 quality metrics (n=3, 13%)24 26 37 and cost-effectiveness of the programme (n=1, 4%),40 but there was no evidence that this monitoring process was routinely performed or overseen by any parties.
Discussion
Summary of evidence
To our knowledge, this is the first scoping review to summarise what is known about CoCE in healthcare.
Despite identifying numerous CoCE initiatives, we were unable to identify selection processes used in more than half of the included initiatives. When selection processes were documented, they varied between initiatives. Further, there were inconsistencies in monitoring CoCE performance. Without consensus on what defines a CoCE, and without a recognised body to monitor the performance within each CoCE, there is no guarantee that care being delivered by sites claiming to be CoCE are delivering excellent (or even better-than-usual) healthcare.
The most common defining feature of CoCE included in this review was resource availability, specifically personnel, infrastructure and equipment. These findings are not surprising; it is well established that there are associations between staffing levels, skill mix, infrastructure and patient outcomes.58–62 For example, higher nursing staffing levels and employment of more skilled staff are associated with better patient outcomes such as reduced rates of pressure injuries, mortality and falls.58 60 Features such as infrastructure and specialised expertise are also key factors in centres of excellence in other industries.8 The inclusion of these features within CoCE reinforces that the included CoCE were designed to align with what is known about healthcare delivery that leads to improved patient outcomes.
While frameworks or processes used to establish or describe CoCE may be valuable to guide others in the field, they may have limitations if these processes were developed for a specific healthcare facility, stakeholder cohort or disease group. For example, the Willis-Knighton Health System is a not-for-profit healthcare network in Louisiana, USA, that operates 11 self-nominated centres of excellence. The framework used to establish these centres of excellence was described by Elrod and Fortenberry and cited by authors of 8 initiatives in our review to describe or establish their centres. Consideration should be given as to whether this framework is fit for purpose beyond the state of Louisiana and in countries with different healthcare models from the USA. Additionally, it is unclear whether this framework meets a universally agreed definition of excellence in healthcare. Empirical research to define ‘excellent care’ from the perspectives of patients, healthcare facilities or funders could increase the validity of the frameworks and, subsequently, the CoCE. A recent study (published after our review was completed) has identified defining criteria of ‘aspirational’ (vs pragmatic, feasible or cost-effective) CoCE in stroke recovery and rehabilitation from the perspective of healthcare providers, survivors and caregivers and researchers. These criteria and the underpinning indicators could be used by facilities seeking recognition as CoCE in stroke healthcare provision.63
Selection procedures for CoCE were inconsistently reported and were unavailable for nearly half the included initiatives. The description of excellent care provided by the CoCE varied, seemingly depending on the agency responsible for defining it. Descriptions of excellence encompassed patient-centric outcomes (eg, optimising clinical outcomes and quality of life), service-centric outcomes (eg, staff skill development, resource availability and meeting quality and safety accreditation) and economic outcomes (eg, cost of treatment and length of stay). The concept of excellence was sometimes conflated with high volume of patients who received care at the centre. Excellence for some centres from low-income and middle-income countries was defined (either by self-nomination or by the government or collaborating international institutions) in providing a particular healthcare service when none was previously available in the region. Many of these aspects of excellence reflect commonly measured quality indicators of healthcare in high-income countries, namely effectiveness, access, safety and efficiency.64 However, cost is not included as a quality metric in countries such as Australia, Canada or the UK, but it is included as a measure of quality in the US Commonwealth Fund framework.64 The difference between healthcare systems that generate income and those that do not is likely to influence many aspects of excellence. The inclusion of cost as a feature of some CoCE could be reflective of the different funding models (eg, fee-for-service vs universal healthcare) or healthcare priorities within the centres or by the bodies determining a site’s excellence. While cost is considered in universal healthcare funding models, it is rarely highlighted beyond ensuring that healthcare providers function within their budget, which markedly differs from financial models that seek to produce profit in fee-for-service healthcare systems. Indeed, the centres that reported economic outcomes as a measure of clinical excellence were predominantly located in the USA and were nominated by healthcare funders suggesting that cost and cost efficiency is overtly considered as an important facet of excellence in fee-for-service centres.65 66
Benchmarking is a well-recognised process that identifies the best-performing healthcare facilities in terms of patient outcomes and system performance.67 However, while there is an implicit assumption that CoCE will deliver care that is superior to another (non-excellent) centre, most of the included initiatives in our review did not benchmark with other services. Benchmarking allows tracking of performance over time while comparing performance against other facilities, thereby demonstrating what is feasible to achieve in terms of quality of care.67 For the initiatives included in this review, without comparison to other healthcare facilities and without a standardised set of explicit, evidence-based and measurable criteria, it raises disparity and challenges on how these centres can claim to be legitimate CoCE.
It is recognised that healthcare performance can be variable,67 so healthcare facilities should monitor and evaluate their programmes to ensure continued excellence. This process needs to be feasible within the time and resource constraints. Just over half the initiatives included in this review reported monitoring their service and described various processes including measuring patient outcomes, service productivity and quality metrics to maintain the designation of CoCE. Only six initiatives reported a structured process, where their ongoing performance was reviewed and assessed by an overseeing body to maintain their status as CoCE. Clearly, more attention should be paid to demonstrate the sustainability of excellence initiatives.
Conclusion
Although CoCE are increasingly reported in the literature, there are inconsistencies in how these CoCE are established, monitored and evaluated. Processes used range from self-designation with no explicit criteria to using external evaluation and periodic recertifications. Features of CoCE centred around skilled medical and multidisciplinary teams and other resources such as infrastructure and equipment. More work is required to develop transparent systems and processes to ensure that centres claiming to be ‘excellent’ can demonstrate that they are delivering the highest quality care.
Implication for practice and future research
This review highlights the need for clear criteria healthcare facilities can use to identify or establish a CoCE. The processes used also need to be transparent so they are easily available for certification or auditing purposes. The concept of a healthcare centre promoting ‘excellence’ can also vary depending on different perspectives: patient, systems or funding. There needs to be clear guidelines that highlight the impact of ‘excellence’ from these perspectives to ensure transparency on why a centre was nominated as a CoCE, and the monitoring processes used. It is recognised that staff well-being and retention contribute to more consistent healthcare delivery and better patient outcomes, so including staff well-being in a CoCE framework may be of value. The findings from this review will contribute to international efforts to establish CoCE using robust, transparent criteria and key performance indicators.
Strengths and limitations
The strengths of our scoping review include the inclusive search strategies (peer-reviewed journals and grey literature) and stringent review process using two independent reviewers throughout the process. There is a potential that there may be established CoCE that have not published any studies or reports, which we then have not identified. While we sought assistance from an academic librarian to ensure the search strategies were clear and comprehensive, centres that describe excellence using different terms and relevant information published in non-indexed sources may have been missed. This is a particular challenge of this focus of work which straddles healthcare organisation, clinical practice and academic research.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
References
Footnotes
X @rachel_stockley, @NatFiniPhysio
Contributors All authors were involved in the screening of records and data extractions. TK was the main author of this work and was responsible for the study design and coordination of the team. TK and EAL were responsible for drafting the manuscript and all authors helped with the critical review of the manuscript. TK is the guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.