Article Text
Abstract
Objective Colorectal cancer is primarily treated with surgery. Major surgery and older age are risk factors associated with postoperative decline in cognitive function. In clinical research, a wide range of instruments have been used to assess cognitive function. There are no clear criteria for the measurement of postoperative cognitive dysfunction. This scoping review aimed to map how and when cognitive function has been assessed after surgery for colorectal cancer and the reported incidence of postoperative cognitive decline.
Design Systematic scoping review following the JBI approach.
Data sources Scopus and PubMed. Last search January 2023.
Eligibility criteria Reports with outcomes of postoperatively assessed cognitive function in colorectal cancer patients with first assessment within 1 year of surgery were included.
Data extraction and synthesis Data were extracted by one researcher and controlled for accuracy by a second researcher. Data were summarised in tables and charts.
Results In total, 49 reports were included (16 clinical trials, 33 cohort studies). Cognitive function was assessed with patient-reported outcome measures, clinical screening tools, neurophysiological testing and complication classification. The definition was most often related to the specific instrument, as predefined cut-off or change from baseline. Assessments were performed between 1 hour and 36 months after surgery—few reports included follow-up both within and after 30 days postoperatively. Incidence of cognitive decline varied considerably (0%–64%), depending on the instrument, definition criteria and time of assessment. Most studies reported a decline in cognitive function after surgery with recovery during follow-up.
Conclusions This study showed a heterogeneity in the choice of assessment method and measurement criteria for cognitive dysfunction after colorectal cancer surgery. A more unified measurement approach in further research would be beneficial to evaluate postoperative cognitive function and understand its impact on the daily lives of patients with colorectal cancer.
Trial registration number 10.17605/OSF.IO/2M3DT.
- Colorectal surgery
- Delirium & cognitive disorders
- Systematic Review
Data availability statement
Data are available upon reasonable request. Data set available upon request, metadata available in supplement.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTH AND LIMITATIONS OF THIS STUDY
This review is following a systematic approach with a preregistered protocol.
Search strategy was developed, and searches conducted by experienced librarians.
There was no critical appraisal for methodological limitation or risk of bias assessment preformed for included studies.
Introduction
Cognitive functions, such as memory, attention and executive functions, can decline after surgery.1 The pathogenesis is not entirely known but most probably it is multifactorial. This can incorporate patient-related factors, including genetic predisposition, the anaesthetic and surgical procedure and the systemic inflammatory response that surgery gives rise to.2 Older age is a risk factor,1 2 but 30%–40% of all adults have been reported to develop postoperative cognitive dysfunction or decline (POCD) after major non-cardiac surgery.3 Generally, it seems to be a temporary condition,2 but patients older than 60 years have an increased risk of persistent cognitive dysfunction 3 months after surgery.3 Colorectal cancer is one of the most common types of cancer worldwide and is primarily treated with surgery.4 5 Considering the high incidence of colorectal cancer, particularly among older adults, a substantial number of patients could be at risk for developing cognitive dysfunction after surgery.
POCD is a research construct and there has been no standardised definition.2 6 In 2018, the international and multidisciplinary Nomenclature Consensus Working Group published a recommendation on cognitive changes after surgery.6 The group aimed to align the terminology of postoperative changes to that of clinical classification of cognitive function in general. The recommended terms were delayed neurocognitive recovery in case of occurrence during the first 30 days after surgery and between 31 and 365 days after surgery postoperative neurocognitive disorder. They further recommended the use of the Diagnostic and Statistical Manual for Mental Disorders’ (DSM-V) criteria for neurocognitive disorder. For diagnosis, DSM-V requires subjective complaints as well as objective testing and specifies that everyday living is hindered at least in terms of instrumental activities (eg, taking medication and paying bills).7 For classification, DSM-V also states that cognitive deficits can not be present solely as a component of delirium.
The assessment of the patients’ function after surgery is an important issue since postoperative recovery, of which cognitive function is an integrated part, is prognostic for long-term recovery and has economic implications.8 A long-term follow-up of a Danish cohort found that patients who developed POCD after non-cardiac surgery retired earlier from the labour market and incurred higher social transfer payments.9 It has also been found that those with POCD at discharge had higher mortality within 30 days and those with persistent dysfunction after 3 months had higher mortality during the first year after surgery.3 While cognitive screening is recommended in American Cancer Society’s survivorship care guidelines for colorectal cancer, it is only mentioned in association with chemotherapy.10 As cognitive decline is associated with major surgery in general, it is reasonable to expect that cognitive decline can occur in patients with colorectal cancer undergoing surgery even if chemotherapy is not part of the treatment regime.
The objective of this review was to map how cognitive dysfunction has been defined and assessed after surgery for colorectal cancer. The aims were to identify research reports of cognitive function after colorectal cancer surgery, explore the incidence of cognitive changes, clarifying the definitions and criteria used and describe how cognitive function has been assessed. The review questions were identified as:
How and when was cognitive function assessed after colorectal cancer surgery?
What definition and nomenclature were used to describe cognitive changes?
What outcome of cognitive function was reported after surgery?
The investigative and explorative nature of the research made it suitable for using a scoping review approach. At the start of this project, we found no registered protocol for systematic reviews at PROSPERO for the assessment of cognitive dysfunction after colorectal surgery, nor any scoping review registered at Open Science Framework. No published protocols or reviews were found on the subject when searched in PubMed, Scopus, Cochrane Database of Systematic Reviews and JBI Evidence Synthesis.
Methods
The protocol based on the JBI methodology11 containing the objectives, inclusion criteria and methods for this scoping review was registered on 24 July 2021 at Open Science Framework, DOI 10.17605/OSF.IO/2M3DT. The registration was made before the screening of results had begun.
The Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) was followed.12 The checklist is available in Supplement I. Patients or the public were not involved in the design, conduct or reporting of this review.
Inclusion criteria
The review included reports on primary research studies. The languages were limited to English and the Scandinavian languages (Norwegian, Swedish and Danish). No restrictions were applied based on the year of publication.
Population was adults with colorectal cancer, the concept examined was outcomes of cognitive function within the context postoperative assessment the first year after cancer surgery.
The criterium of assessment within 1 year was added after protocol registration to align with the recommended temporal specification for postoperative cognitive changes, that is, only in the first 12 months after surgery.6
Search strategy
The main search was conducted by librarians at the Biomedical Library, University of Gothenburg, on 23 April 2021, in PubMed (via Medline) and Scopus databases. A subsequent search was made on 3 January 2023.
Search on Scopus:
TITLE-ABS-KEY (pocd OR ‘PostOperative Delirium’ OR ‘postoperative decline’ OR ((cognitive OR neurocognitive OR memory) W/3 (postoperative OR complication* OR decline OR dysfunction OR disorder* OR recovery OR impairment OR sequelae OR frailty)))
AND
TITLE-ABS-KEY ((colonic OR colon OR colorectal OR rectal) W/3 (neoplasm* OR cancer* OR tumour* OR tumour* OR surgery)).
In addition to database searches, bibliographic searches were conducted. Excluded review articles that contained key terms in the title (colorectal cancer or surgery, cognitive function or effects of cancer treatments) were scanned for relevant sources. This was repeated for all reports included in the full-text examination. The complete database search strategy is available in online supplemental file II.
Supplemental material
Screening and selection
After the removal of duplicates, search results were transferred to the web-based screening tool Rayyan.13 Two blinded reviewers screened titles and abstracts. Conflicts were discussed, and the senior author had the last say if a consensus was not reached. Full-text screening was performed by one researcher in EndNote.14 Exclusion criteria for all excluded reports were confirmed by another researcher.
The exclusion criteria for screening had no hierarchy, and the first relevant exclusion criterium was used for classification. Predefined reasons for exclusion in the title and abstract examination were protocol or review, not primary research and no participants with colorectal cancer or surgery. During the screening process, the following exclusion criteria were added; metastatic surgery (including hyperthermic intraperitoneal chemotherapy) and focus on effects of chemotherapy on cognitive functions since it is not relevant to primary colorectal surgery; delirium assessed only by a clinical definition (ie, no cognitive testing) and no assessment within 1 year of surgery. Case-reports were excluded.
Data charting
Data were extracted by one researcher. For the initial search, the software NVivo15 was used in qualitative and iterative process to categorise text and figures depending on content relevant to the review questions. Data were then charted in an Excel spreadsheets using Colectica16 for metadata. For the subsequent search, data were charted directly to the spreadsheet. The results were then compiled into relevant tables and charts. All charted data were controlled for accuracy by a second researcher.
Data were charted for study characteristics such as aims, methodology and study population. Data relevant to review questions were nomenclature, definitions and instruments used. The time of assessment was charted as months, days or hours as specified in each report. Cognitive outcomes were charted as frequency and if decline and recovery occurred and differences between compared groups. Since not all reports used statistical testing for within-group comparison, numerical values were compared as presented. Details of all charted variables used in this review are presented in the metadata in online supplemental file III.
Supplemental material
Results
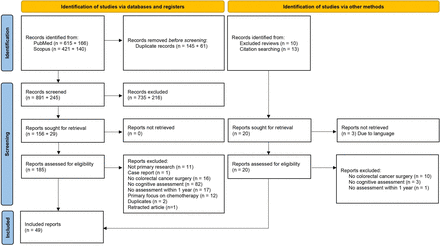
After the removal of duplicates, 1136 records were screened in title and abstract examination. There were 23 reports identified from other sources (figure 1).17 Out of the 205 articles that were subjected to full-text examination, 49 were included. Online supplemental file IV includes a summary of data relevant to the review questions from all included reports.
Supplemental material
PRISMA flow chart. PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses.
The included reports were published during 2000–2022. There were 33 observational cohort studies and 16 reports of controlled trials. The aim of reports was mainly to investigate cognitive function (39%), quality of life (41%) or recovery after surgery (14%). Table on characteristic for all included sources is in online supplemental file V.
Supplemental material
Thirty-nine study populations were exclusively patients with colorectal cancer, in the remaining study populations, colorectal cancer patients comprised 19%–89%. Sample sizes in observational studies ranged 11–1129 and in clinical trials 40–281. Across all studies, there was a mean of 46% female participants, and the average age reported was 66 years, covering a range of 18–99 years. The study populations were mainly from Europe (47%) and Asia (43%), the remaining reports had populations from Australia, Brazil, Canada and USA. There was also one international online population.18 In five reports, the participants had received no other cancer treatment than surgery.19–23 Information on adjuvant treatment was given in 20 reports.
Perioperative intervention concerning anaesthesia (types of drugs or procedural aspects) was used in 81% (n=13) of the clinical trials with dexmedetomidine being used in half of those (n=6). Observational studies compared groups most frequently according to surgical method or procedure (n=8), healthy controls or the general population (n=6), patients’ age (n=5) or whether postoperative cognitive decline developed or not (n=5).
Assessment of cognitive function
Cognitive function was generally assessed with questionnaires or screening tools (figure 2). The two other assessment methods were neuropsychological testing and complication classification. More than one type of assessment method and instrument could be used in the same report. See online supplemental file VI for full list of instruments. A separate assessment of postoperative delirium was made in eight reports,19 24–30 and instrumental activities of daily living (IADLs) were reported after surgery in two sources.27 31
Supplemental material
Graph of instrument for assessment of cognitive function. EORTC QLQ-C30, European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire—Core 3.0; MMSE, Mini-Mental State Examination.
A total of six questionnaires, five previously described and one novel18 was used for patient-reported outcomes. Answers to questionnaires were collected by in person or telephone interviews or self-administered during visits, online or at home. The European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire—Core 3.0 (EORTC QLQ-C30) was the most frequently utilised instrument overall. Studies that utilised patient-reported outcomes were generally observational studies with focus on quality of life. One clinical trial used self-reported outcomes of cognitive function.32
Five different screening tools were represented with the Mini-Mental State Examination (MMSE) as the most utilised. When specified, screenings were done by trained personnel, often the same individual for all assessments, and with the assessor blinded to the patient’s intervention group. Screening tools were used in all but two clinical trials. In reports with the aim to investigate cognitive function screening tools were the most frequent instrument employed (12/19).
Two reports measured cognitive dysfunction as a complication, both were observational studies reviewing patient records and grading with the Clavien-Dindo classification.33 34 Nine reports assessed cognitive functions with neuropsychological testing employing a wide range of tests for several cognitive domains such as processing speed, attention and verbal memory. Tests could be used either together as a battery with a composite score or as individual tests, reported separately. The time requirement for neuropsychological testing was given in three reports, 30, 60 and 90 min. When reported, testing was done in a quiet environment and by trained personnel. There were two computerised tests, the Attention Network Test (ANT) and the Cambridge Neuropsychological Test Automated Battery (CANTAB). Neuropsychological testing was used in three clinical trials, once as the only assessment method28 and otherwise in combination with a screening tool.25 35 When reported separately return to preoperative values occurred later when assessed with neuropsychological testing than with screening tool.25 In one case both CANTAB and a battery of seven individual neuropsychological tests were uses in the same report23 and the association between the neuropsychological testing methods was stated as weak-to-moderate.
Across studies, cognitive assessment was performed in the shorter term, 1–12 hours and 1–30 days after surgery, and in the longer term, 2–36 months after surgery. Most reports had a follow-up only within 30 days (49%) or only after 30 days (41%). One clinical trial had follow-up after the first 30 days.32 Cognitive function was assessed up to 11 times, including baseline, with a mean of three assessment points. There were six cross-sectional reports.
Nomenclature and definition
Impairment was the most frequent term used to describe cognitive function decrease in general, followed by dysfunction, both terms occurred in several combinations. Neurocognitive was used in combination with impairment, decline, deficit and dysfunction. About half of the reports utilised more than one term. Two reports referred to problems with concentrating and memory without any generic term. Sixteen reports used cognitive or mental function, capacity, or ability without any term indicating a decline in function.
A narrative definition of POCD as a concept was absent in most reports. When present, it concerned the decline of cognitive functions such as memory, executive control and attention. Two reports also mention decline in social ability.36 37 Four reports included symptoms such as confusion, disorientation, anxiety, agitation or delirium in their definition.22 36 38 39 Two reports stated that no abnormalities in cognitive function should have been present preoperatively.35 39
A little more than half of the reports presented criteria for measurement of cognitive dysfunction. Instrument-specific criteria were most common. Both predefined cut-offs and change from baseline was used, with or without subdivisions. Instrument-specific criteria were used with screening tools and questionnaires, for neuropsychological testing, general criteria were more common (table 1). The Z-score was the most common general criteria, defined in four reports. Occurrence of specific or any symptoms of cognitive decline was also used as criteria both with questionnaires and complication classification. There was also a vague definition (ie, the lower the score, the lower the function).
Criteria for measurement of cognitive dysfunction
Outcome of cognitive assessments
Of the reports that had comparable preoperative values, 86% (30/35) showed a decline at the first follow-up after surgery. The reports not showing decline had follow-up at 1 month as the earliest.24 40–43 Of the reports showing decline, one-third (10/30) had first follow-up after the first 30 days. Full or partial recovery occurred in most reports (figure 3). Recovery occurred at the earliest 1 day after surgery and at the latest after 24 months. In four reports, no recovery occurred within the follow-up period (5 days–12 months).19 31 35 44 In seven reports, there was a decline of function after a previous assessment had shown recovery.
Graph of recovery within follow-up period.
Incidence of cognitive dysfunction after surgery
The frequency of cognitive dysfunction after surgery was presented in 20 reports. Across these, the instruments for assessment, measurement criteria for dysfunction and follow-up periods differed (table 2). Postoperative incidence ranged from 0% to 64%, incidence of cognitive dysfunction at baseline was reported in three reports, 8.2%–28%.
Reports with frequency of cognitive dysfunction
There were eight clinical trials presenting incidence, most of them had one assessment within 7 days of surgery (table 2a). The highest incidence reported was 64%, which represented a total of patients with cognitive dysfunction at postoperative days 1 and 3 in a control group.39 A 0% incidence was reported 3 days after surgery in an experimental group.22 Across all reports, the incidence ranged 8.8%–25% at the earliest follow-up, 1 day after surgery. At 7 days after surgery an incidence of 5%–29% was reported across all reports. All reports with more than one postoperative follow-up showed decreasing numbers of cognitive dysfunction over time. One study reported baseline incidence of 16%–17%, at follow-up, 30 days after surgery, the incidence was lowered in the intervention group and increased in the control group.27
For the observational studies (table 2b), the highest incidence was 56%, reported in a cross-sectional report 12 month after diagnosis.45 The remaining reports with data for 12 months had an incidence between 2.7% and 49%. The lowest incidence reported was 1.8% as a total within 30 days of surgery.34 At 7 days after surgery, an incidence of 25%–34% was reported across all studies. In the reports with more than one postoperative assessment, incidence generally decreased with time. At the latest follow-up, around 2 years after surgery, incidence ranged 17%–29% across reports. One study reported incidence for older persons without cancer as 22% which was stable after 12 months, while the incidence increased for cancer patients.31 A cross-sectional report showed differences in incidence with neuropsychological testing but not with self-reported measures when comparing cancer patient to healthy controls.23
Discussion
The 49 reports in this review assessed cognitive function after surgery using a diversity of methods and definitions. Due to the heterogeneity across definitions and assessment methods, it is difficult to synthesise information, and reach firm conclusions regarding incidence of cognitive decline after colorectal cancer surgery. Nevertheless, decline in cognitive function was found in more than 80% of the reports with preoperative levels, regardless of the instrument and the specific definition. Collectively, the data suggest that changes in cognitive function do occur in colorectal cancer patients who received surgery.
A limitation of this study, as inherent with all reviews, is the possibility that some relevant sources have been missed. However, the findings in this review are consistent with the broader literature. For example the EORTC QLQ-C30 was the most used instrument when measuring cognitive function after chemotherapy in a colorectal cancer population46 and the MMSE is the mostly used screening tool for postoperative cognitive assessment.1 Since this scoping review had an exploratory focus, we did no formal rating of the quality of evidence and therefore any conclusions drawn based on the results of included studies must be made with caution.
A general concern with the data in this review is that a large portion is obtained through self-report or screening tools. Subjective complaints of cognitive function are poorly correlated with objective testing in cancer patients.23 47 It has therefore been suggested that subjective complaints might be an indicator of anxiety and depression rather than cognitive dysfunction.47 48 It is recommended that cognitive changes after surgery should be assessed with neuropsychological tests for specific cognitive domains rather than with screening tools.6 49 Among the reports in this review employing objective measurements, the use of screening tools was twice as common as neurophysiological testing. Of the studies that aimed to investigate cognitive function, fewer than half used neurophysiological tests. There has been discussion on whether screening tools are appropriate or not when detecting POCD,2 for detecting cognitive changes after cancer treatment screening tools are, however, not considered sufficient.50 Another concern with the data is the potential overlap between postoperative decline of cognitive functions and postoperative delirium.6 51 Delirium has its own diagnostic definition, and focuses on awareness and by definition, to diagnose neurocognitive disorder, cognitive deficits cannot be present solely as part of delirium.7 Only eight reports in this review performed a separate assessment of delirium making it uncertain in the other studies whether the cognitive decline reported was delirium induced or not, at least in the period directly after surgery when there is a risk of postoperative delirium.52
Decline of cognitive function in the first 30 days after surgery is defined as delayed neurocognitive recovery in the recommendation on terminology of cognitive change after surgery.6 This period is affected by complicating factors such as delirium, immobility and analgesic medication, such as opioids, which also could give rise to cognitive dysfunction. Patients receiving intensive care have a high risk of developing cognitive dysfunction.53 The need for intensive care after surgery might therefore be related to postoperative cognitive decline soon after surgery. About half of the reports in this review reported only on the period within the first 30 days and with only one of the interventional studies having follow-up after 30 days it is not known if the effects of interventions persist after the recovery window. Overall, it has been questioned if POCD persists over time.2 A recently published study indicates that there is no cognitive impairment in the long term for colorectal cancer survivors.54 It has been suggested that postoperative cognitive function should not be assessed later than 6–9 months after surgery,55 but in the recommendation of terminology, the denotion postoperative apply to new occurrence or deterioration of pre-existed impairment up to 12 months after surgery.6 In this review, recovery of cognitive function was reported in all but a few reports with preoperative values and follow-up after 30 days. Incidence in included reports decline over time. However, the incidence of cognitive dysfunction after surgery might be underestimated during long-term follow-up due to the inability of patients with the worst declines to participate in studies.56 This selection bias could also inflate reports of cognitive recovery since the study population may have a higher mean function over time as those with lower scores cannot continue their participation.
The heterogeneity shown in this review regarding instrument and criteria of measurements are similar to a recent review on cognitive impairment after chemotherapy in colorectal cancer patients46 and has also been shown previously with assessment of POCD.1 2 55 To adhere to a common criterion would be beneficial to synthesise results and to explore what effects postoperative cognitive decline has for patients and in the clinic. How to best measure cognitive function is beyond the scope of this review. However, advocates for patient-focused care have stressed that when assessing recovery after surgery, the patient should act as their own control.8 Measurement criteria using that approach would reduce the risk that a decline in a person with normal high or low function might go unnoticed if they remain above or always was below a predefined threshold for impairment.7 There is of course the discussion of what changes should be considered significant and the point of interest is perhaps better focused on if the functional decline affects the patient’s daily life or not. Assessment of instrumental activity of daily living (IADLs) are considered a good indicator of problems derived from subtle cognitive decline.6 7 Yet only two reports in this review reported IADLs.
As there was no formal rating of the quality of evidence reports included in the scoping review, the overall conclusions are considered to have low evidence. Nevertheless, a majority of the reports in this review noted cognitive functional decline in the study populations with comparable preoperative levels. When it comes to colorectal cancer patients, adjuvant treatments as well as the cancer itself need to be considered as causative factors for cognitive decline.57 A holistic approach to cognitive decline for all colorectal cancer treatments and the cancer itself would surely be beneficial. Therefore, extending recommendation of cognitive screening of patients receiving chemotherapy to all colorectal cancer survivors, regardless of treatment modality, could be of value and requires further investigation, especially considering that the existing recommendation has the lowest level of evidence.10
To strengthen the evidence on cognitive decline after colorectal cancer surgery, neurophysiological testing should likely be considering worth the effort in future research. Future research would also do well to considering separate assessment of delirium. Especially when assessing cognitive function soon after surgery, it has implication also in the long run since there is an indication that those with postoperative delirium are less likely to recover from cognitive changes after surgery.51 Studies assessing both cognitive function and IADL would also provide a more detailed account of how cognitive decline impacts patients’ lives after colorectal cancer surgery. Randomised controlled trials with longer follow-up periods could also be a valuable contribution to provide knowledge on if a perioperative intervention would have effect on persistent cognitive decline.
Conclusion
A more unified approach when it comes to the criteria for measurement of postoperative cognitive function would be beneficial to align research and increase the quality of evidence. Longitudinal studies with follow-up both within and after 30-days, preferable with neuropsychological testing and separate assessment of delirium, would provide new knowledge on whether cognitive dysfunction persist after the recovery period. Randomised controlled trials with the same approach could also contribute with knowledge on whether interventions do reduce actual neurocognitive decline and not only delirium induced manifestation. There could also be room for more research that inform on the degree to which the postoperative cognitive function decline impacts the daily lives of colorectal cancer patients.
Data availability statement
Data are available upon reasonable request. Data set available upon request, metadata available in supplement.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
This work has previously been presented as a poster at Kirurgveckan 2023, Örebro, Sweden and ESCP’s 18th Scientific Conference, Vilnius, Lithuania, 2023.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors CE is responsible for the overall content as guarantor. CE provided concept and protocol, screened based on all examination levels, charted and summarised data, and wrote the manuscript. EA reviewed protocol, screened based on title and abstract examination, revised manuscript and provided clinical and research expertise. RL controlled charted data and exclusion based on full-text examination, and revised manuscripts. All authors read and approved the final manuscript. Non-authors’ contributions: Eva Hessman and Linda Hammarbäck, Biomedical Library, Gothenburg University Library, University of Gothenburg, Gothenburg, Sweden, advised on search strategy, conducted searches and retrieved full texts. Andreas Samuelsson, Scandinavian Surgical Outcomes Research Group (SSORG), screened based on title and abstract examination for subsequent search.
Funding This work was supported by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement, grant number ALFGBG-965084.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.