Article Text
Abstract
Introduction The exact role of hepatic arterial infusion chemotherapy (HAIC) in advanced hepatocellular carcinoma (aHCC) is still unknown. The combination of HAIC and sorafenib has been proven to be more effective than sorafenib alone in the first-line treatment of aHCC. The aim of the study is to evaluate the efficacy and safety of HAIC plus regorafenib in the second-line treatment of aHCC.
Methods and analysis This is a multicenter, open-label, randomised controlled phase III trial. A total of 294 patients with aHCC, who are unable to tolerate the first-line systemic therapy or progress after the first-line systemic therapy, will be enrolled in the study. The patients will be randomly (2:1) assigned into the combination treatment group (HAIC plus regorafenib, n=196) and the control group (regorafenib alone, n=98). HAIC and regorafenib (160 mg/day) will be given in a 4-week cycle. The primary endpoint is overall survival in the intention-to-treat population. The second endpoints include progression-free survival, overall response rate, time to progression, etc. The radiological assessments will be based on the criteria of Response Evaluation Criteria in Solid Tumors 1.1.
Ethics and dissemination This study is approved by the ethics committee of Cancer Hospital, Chinese Academy of Medical Sciences. All participants are required to provide written informed consent. The results of this study will be disseminated through peer-reviewed publications and esteemed academic conferences.
Trial registration number Chinese Clinical Trial Registry (ChiCTR2300073075).
- Hepatobiliary tumours
- Interventional radiology
- CHEMOTHERAPY
- Randomized Controlled Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is the first investigator-initiated, multicenter, randomised controlled phase III trial to investigate the role of hepatic arterial infusion chemotherapy in the second-line treatment of advanced hepatocellular carcinoma (HCC).
The primary endpoint is overall survival, which will be complemented by progression-free survival, objective response rate, time to progression, disease control rate, surgical conversion rate and adverse events.
This study would allow us to clarify the exact role of hepatic arterial infusion chemotherapy (HAIC) in patients with advanced HCC and find the subgroup of patients who can benefit most from the combination of HAIC and regorafenib.
It could be a limitation that regorafenib, not the other drugs developed in recent years for HCC, is chosen to be the standard treatment in the control group.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer.1 2 Advanced HCC (aHCC) represents those with macrovascular invasion, extrahepatic spread or cancer-related symptoms. The median survival time of aHCC is merely 6–8 months without any treatment.3 Multiple first-line (ie, sorafenib, lenvatinib, atezolizumab plus bevacizumab and tremelimumab plus durvalumab) and second-line (ie, regorafenib, cabozantinib and ramucirumab) treatments have been successfully used to improve the prognosis of aHCC.4 5 However, the median overall survival of aHCC is still less than 2 years.3 Therefore, novel treatments are warranted to further improve the prognosis of aHCC.
Hepatic arterial infusion chemotherapy (HAIC) using oxaliplatin, leucovorin and fluorouracil showed better efficacy than transarterial chemoembolization (TACE) in patients with large HCC,6 and HAIC combined with sorafenib has shown better survival outcomes than sorafenib for the first-line treatment of aHCC.7 However, with the rapid development of novel systemic treatments for aHCC, the exact role of HAIC in advanced HCC is still unknown.8–10 To the best of our knowledge, no published phase I or phase II trials have yet evaluated the efficacy of HAIC in combination with regorafenib for the treatment of advanced HCC. Nevertheless, this combination therapy has garnered increasing attention and application in patients with unresectable colorectal liver metastases, where several retrospective studies have demonstrated its effectiveness and tolerability.11 Therefore, we hypothesise that HAIC might be able to improve the efficacy of regorafenib in the second-line treatment of aHCC. Thus, this study aims to compare the efficacy and safety of HAIC plus regorafenib with regorafenib alone for the second-line treatment of aHCC.
Methods and analysis
This investigator-initiated, multicenter, open-label, randomised controlled phase III trial has been approved by the local ethics committee and registered in the Chinese Clinical Trial Registry (identifier: ChiCTR2300073075). A total of 294 patients recruited from three institutions, including Cancer Hospital of Chinese Academy of Medical Sciences, Shenzhen Cancer Hospital and Hebei Cancer Hospital, will be randomly (2:1) assigned into the combination treatment group (HAIC plus regorafenib, n=196) and the control group (regorafenib alone, n=98) (figure 1). The study will commence patient enrollment from August 2023 and conclude this phase by January 2025, with the subsequent follow-up period extending until July 2026. The study protocol is in accordance with clinical practice guidelines, the Consolidated Standards of Reporting Trials statement, the Standard Protocol Items: Recommendations for Interventional Trials 2013 checklist and the Declaration of Helsinki. All patients will provide written informed consent (online supplemental file 1).
Supplemental material
Consolidated Standards of Reporting Trials diagram. HAIC, hepatic arterial infusion chemotherapy.
Inclusion and exclusion criteria
Patients who tolerate first-line systemic therapy or whose tumour is found to progress after first-line systemic therapy will be enrolled in the study.4 5 HCC diagnosis will be determined by biopsy or imaging examinations, according to the criteria of the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases.3 12 Advanced HCC is referred to as being at the Barcelona Clinic Liver Cancer (BCLC) stage C, according to the BCLC staging system.4 Inclusion and exclusion criteria are listed in table 1.
Inclusion and exclusion criteria
Interventions
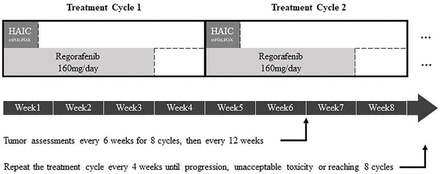
Patients in the combination treatment group will receive HAIC and regorafenib (160 mg/day) in a 4-week cycle (figure 2). And patients in the control group will receive regorafenib (160 mg/day) for the first 3 weeks in a 4-week cycle.13 Interruptions and dose reductions of regorafenib are allowed to manage the related toxicity according to the judgement of the physician. Generally, when grade 3 or higher regorafenib-related adverse events (AEs) occur, regorafenib will be discontinued. If no recovery occurs (ie, if the AEs are not reduced to grade 2 or lower) after a 30-day delay, regorafenib will be discontinued permanently. If the AEs are reduced to grade 2 or lower within 30 days, regorafenib treatment will continue at a reduced dose level (ie, initially 120 mg/day, then 80 mg/day if necessary), and the subject will be closely monitored weekly for at least 4 weeks. For patients in the combination group, HAIC will be administered at the beginning of the first week during the 4-week cycle. After determining and catheterising the intrahepatic tumour blood supply, the microcatheter and sheath will be fixed, and oxaliplatin (85 mg/m2), leucovorin (400 mg/m2), fluorouracil (400 mg/m2) (bolus) and fluorouracil (2400 mg/m2) (mFOLFOX6) will be sequentially infused through the microcatheter in the next 48 hours.14 HAIC treatment will be repeated every 4 weeks until (1) intolerable serious adverse reactions occur (continued HAIC according to the drug reduction plan if the adverse reactions decrease to level one or two within 28 days and permanent discontinuation of HAIC if it cannot be reduced to level one or two after 28 days of discontinuation), (2) tumour progression, (3) death, (4) patient withdrawal of the informed consent or (5) other situations that the physician deems it necessary to stop HAIC. Concomitant best supportive care is allowed during the trial. After eight treatment cycles, patients are allowed to use any treatment that the clinical practice guidelines allow. It is acceptable for patients in the regorafenib alone group to use HAIC as a treatment after tumour progression.
Timeline of the treatments. Patients in the combination treatment group will receive both HAIC and regorafenib treatment. And the patients in the control group will receive regorafenib alone. HAIC, hepatic arterial infusion chemotherapy.
Outcomes
The primary endpoint is overall survival, which is defined as the time between the date of randomisation and the date of death (or the date of the last follow-up when the patient is alive). The secondary endpoints include overall progression-free survival, hepatic progression-free survival, objective response rate, time to progression, disease control rate, surgical conversion rate and AEs.6 The radiological assessment will be independently centralised and reviewed by investigators using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria, and the outcomes derived from mRECIST criteria will also be recorded. AEs after entry into the study will be recorded in line with the Common Terminology Criteria for Adverse Events V.5.0. Quality of life will be recorded using the quality-of-life questionnaires for hepatocellular carcinoma (QLQ-HCC18) and cancer (QLQ-C30) released by the European Organisation for Research and Treatment of Cancer.
Data collection and follow-up
Age, gender, underlying liver disease aetiology, contrast-enhanced CT/MRI scans, tumour marker testing, blood routine analysis, coagulation function assessment, liver function tests, Eastern Cooperative Oncology Group (ECOG) performance status and an assessment of quality of life will be collected at patient enrollment. Follow-up visits for tumour assessment, including contrast-enhanced CT/MRI, tumour marker testing, blood routine, coagulation function, liver function tests and AE monitoring, are scheduled every 6 weeks for the first eight treatment cycles and every 12 weeks thereafter, until death or the end of the study. Double data entry will be used to enhance the data quality. To promote participant retention and complete follow-up, e-mails or mobile messaging will be sent to the participants, reminding them of the upcoming data collection. Files of the participants will be stored in numerical order and stored in a secure place. The files will be stored for 3 years after the end of the study. All electronic databases will be secured with password-protected systems.
Randomisation
The patients will be randomly assigned (2:1) to receive regorafenib with or without HAIC by the method of stratified blocked randomisation.15 The stratification factors include the ECOG performance status (0 vs 1), macrovascular invasion or extrahepatic disease (yes vs no) and prior systemic treatment (lenvatinib vs atezolizumab plus bevacizumab vs others).3 The length of the blocks is also randomly determined by a computer and concealed to all investigators. The statistician will not be involved in the enrollment.
Blinding
Due to the nature of the intervention, both the participants and the investigators will be aware of the treatment assignments after the randomisation. The data collectors, statisticians and the independent radiologists who conduct the imaging review will be blinded to the allocation. An employee outside the team will input related data into the computer so that the investigators can analyse these data without having access to the information of allocation.
Statistical analyses
The primary and secondary endpoints between the two groups will be compared by intention to treat. Continuous variables will be compared by Student’s t-test or Mann-Whitney U test as appropriate. χ2 or Fisher’s exact test will be used for categorical variables. The analyses of safety will be based on the patients receiving at least one full cycle of the assigned treatment. Subgroup analyses will be conducted according to age, sex, ECOG performance score, prior systemic treatment, macrovascular invasion, extrahepatic disease, alpha fetoprotein level, hepatitis B infection and hospitals. Kaplan-Meier survival analysis followed by multivariable Cox proportional hazards model will be used to compare the timed endpoints such as overall survival. Comparisons between the per-protocol groups will also be conducted to validate the results derived from the intention-to-treat groups. A two-sided p value<0.05 will be considered statistically significant. No interim analysis is planned.
Sample size
The median overall survival time of patients treated with regorafenib alone is estimated to be 10 months, according to previous studies.13 16 A 50% increase in median overall survival time (ie, 15 months) in patients treated with HAIC plus regorafenib is expected, with a HR of 0.67. To detect the difference with 80% power and a two-sided α of 0.05, a total of 294 patients are needed, with an enrollment period of 18 months, a total study period of 36 months and a drop-out rate of 10%. Patients will be recruited at the study enters through two methods: (1) local advertising and (2) identification in the outpatient clinic.
Data monitoring and quality assurance
Monitoring visits will be scheduled every month to confirm the integrity and accuracy of the data in the electronic system by comparing it with the data in the original documents.
Reporting of the trial results
The results will be released to participants, investigators and the medical community. Abstract or article derived from the data of the current study must be reviewed and approved by the investigators about its appropriateness before submission.
Patient and public involvement statement
Before submitting the study protocol to the local ethic committee, investigators collaborated with three patient representatives with aHCC to comprehensively assess and improve the protocol. Their feedback was meticulously incorporated into the present trial design by making necessary clarifications and corrections.
Discussion
According to the Barcelona Clinic Liver Cancer staging system and recent guidelines, second-line treatments of aHCC include regorafenib, cabozantinib, lenvatinib, sorafenib, pembrolizumab, ramucirumab, etc.3–5 Lenvatinib and sorafenib are still widely used in patients seeking first-line treatment of aHCC due to their convenience and low price, especially in China.17 18 And as of July 2023, cabozantinib, pembrolizumab and ramucirumab have not been listed in the national health insurance drug catalogue of China. Therefore, regorafenib is chosen as the standard treatment in this investigator-initiated study.
FOLFOX-HAIC has been proven to be more effective than TACE in patients with large HCC, and HAIC combined with sorafenib has shown better survival outcomes than sorafenib alone for the first-line treatment of aHCC.6 7 In addition, HAIC is relatively more cost-effective than Yttrium-90 hepatic radioembolisation for patients with aHCC in China. In addition, HAIC plus regorafenib has been proven to be safe in advanced colorectal cancer in a real-world retrospective study, in which grade 3 or 4 AEs were observed in seven (18.4%) patients and two patients discontinued the combination treatment because of AEs.11 To further reduce the AEs of the combination therapy, the interval for conducting HAIC is extended from 3 weeks to 4 weeks. An extra advantage of this extension is that the treatment cycle of HAIC can be coordinated with the treatment cycle of regorafenib, which is more convenient for the physicians to monitor adverse reactions and for patients to follow the treatment plan. Thus, we regard that the combination of HAIC and regorafenib in a 4-week cycle might be the optimal second-line treatment for aHCC.
In conclusion, this randomised controlled trial would allow us to clarify the exact role of HAIC in patients with aHCC and find the subgroup of patients who can benefit most from the combination of HAIC and regorafenib.
Ethics and dissemination
This study is approved by the ethics committee of Cancer Hospital, Chinese Academy of Medical Sciences, and registered in the Chinese Clinical Trial Registry (identifier: ChiCTR2300073075). All participants are required to provide written informed consent. The results of this study will be disseminated through peer-reviewed publications and esteemed academic conferences.
Ethics statements
Patient consent for publication
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors HZ contributed to the design, statistical analysis and writing of article. XZ, PT, YL, WS, YL, JL, TG and ZY contributed to the discussion of the protocol and data acquisition during study. PS contributed to the discussion of the protocol and critical revision and was the head of the data management team. XL contributed to the concept and design and study supervision and critical revision and was the head of the steering committee. XL is the guarantor of this study.
Funding This work was supported by the National Natural Science Fund of China (Grant number: 82001937, 82330061) and the CAMS Initiative for Innovative Medicine (Grant number: 2021-I2M-1-015-3). The providers of these funds have no role in any activities of the study.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.