Article Text
Abstract
Introduction Almost a quarter of patients with tuberculosis (TB) in Pakistan may also have diabetes, with an additional quarter in a pre-diabetic state. Diabetes is a risk factor for TB. When it co-occurs with TB, it leads to poorer outcomes for both conditions, considerably increasing the burden on individuals, families and the healthcare system. We aim to improve health, quality of life and economic outcomes for people with TB and diabetes by optimising diabetes prevention, screening and management within TB care. The objectives of this study are to: (1) design an integrated optimised tuberculosis-diabetes care package (Opt-TBD) and its implementation strategies; (2) pilot and refine these implementation strategies and (3) implement and evaluate the Opt-TBD care package in 15 TB care facilities in Pakistan.
Methods and analysis We will work with the TB programme across two provinces of Pakistan: Khyber Pakhtunkhwa and Punjab. TB care facilities will be selected from urban and rural settings of these provinces and will include three levels: primary, secondary and tertiary care settings. This multiphase mixed-method study has three sequential phases. Once ready, the care package and its implementation strategies will be piloted to inform further refinement. The package will be implemented in 15 urban and rural TB care facilities and evaluated for its Reach, Effectiveness, Adoption, Implementation and Maintenance, and potential for scale-up. Quantitative data will assess provider adoption and the package’s accessibility and effectiveness for patients with TB and with diabetes and pre-diabetes. Qualitative data will explore barriers and facilitators for successful implementation and scale-up. Data will be analysed using statistical methods—including descriptive and inferential statistics—for quantitative data and framework analysis for qualitative data, with triangulation to integrate findings.
Ethics and dissemination Ethics approval was granted by the National Bioethics Committee of Pakistan (NBCR-1010). Findings will be shared through academic publications, conferences and public outreach.
- Tuberculosis
- Diabetes Mellitus, Type 2
- Implementation Science
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study employs a multiphase mixed-methods design, which combines qualitative and quantitative approaches to provide a comprehensive evaluation of the implementation and outcomes of the integrated tuberculosis (TB)-diabetes care package.
The study incorporates multiple established frameworks, including the WHO Health Systems Framework, Expert Recommendations for Implementing Change Framework, Proctor’s Outcome Framework and Reach, Effectiveness, Adoption, Implementation and Maintenance Framework, ensuring that the implementation strategies are systematically developed, assessed and tailored to the healthcare context in Pakistan.
The use of co-design workshops, involving healthcare providers, patients and caregivers in shaping the intervention, ensures that the care package is both contextually relevant and patient-centred, improving the likelihood of successful implementation.
The study is limited by its geographic focus on two provinces in Pakistan, which may restrict the generalisability of findings to other low- and middle-income countries with different healthcare infrastructures or socioeconomic factors.
Potential variations in data quality and consistency across different healthcare facilities and laboratory settings may affect the accuracy of TB-diabetes diagnosis and care implementation, though mitigation strategies such as training and quality checks are in place.
Introduction
The co-occurrence of tuberculosis (TB) and diabetes presents a significant challenge in low- and middle-income countries (LMICs) like Pakistan.1 Pakistan is ranked fifth among high TB burden countries, reporting approximately 510 000 new TB cases2 and 56 000 TB-related deaths annually,3 with the most recent estimate of 1180.8 Disability-Adjusted Life Years (DALYs) per 100 000 population.4 Concurrently, diabetes is also common in Pakistan; 17% of adults may have diabetes and another 11% pre-diabetes,5 leading to 1135.59 DALYs per 100 000 population.4 Bearing a substantial burden of TB and diabetes, nearly a quarter of patients with TB in Pakistan may also have diabetes, with an additional quarter in a pre-diabetic state.6 Diabetes is a risk factor for TB.7 When it co-occurs with TB, it leads to poorer outcomes for both conditions, considerably increasing the burden on individuals, families and the healthcare system.8 Integrating diabetes care within TB care can potentially reduce this burden and save healthcare costs while offering more patient-centred, high-quality care.9 10
WHO advocates for integrated healthcare interventions for comorbidities,11 12 but evidence on implementation is limited, especially in low-middle income countries.13 14 A systematic review suggests participatory approaches for complex interventions targeting specific comorbidity clusters.15 Feasibility studies have been conducted on screening and managing TB and diabetes comorbidity, particularly in Pakistan.6 13 These studies have shown promise in detecting previously undiagnosed diabetes among patients with TB and improving diabetes control. Yet, a definitive body of evidence on the implementation, effectiveness, equity and sustainability of integrated approaches is lacking.
The urgency of effective TB control is underscored by the Sustainable Development Goal (SDG 3.3) and WHO targets for reducing TB incidence and mortality by 2030 and 2035, respectively.16 17 However, achieving these goals depends on addressing comorbid conditions such as diabetes. In Pakistan, failing to manage the rising prevalence of diabetes within TB care could significantly impede efforts to reduce TB incidence. Pakistan’s well-structured TB programme presents a unique opportunity to integrate diabetes prevention and management within TB care, contributing to achieving both TB and broader health-focused SDG targets. While the global call for integrated care is crucial, there is currently insufficient LMIC-based evidence on how to integrate and implement TB and diabetes care effectively. Given the high burden of TB and diabetes in Pakistan, integration is urgently needed to optimise resource allocation and improve clinical outcomes for both conditions.
Despite Pakistan’s well-established TB control programme, care for individuals with comorbid TB and diabetes remains fragmented and lacks personalisation.18 Critical opportunities for screening, prevention and management of diabetes are frequently overlooked.18 Consequently, diabetes often remains undiagnosed and untreated among this high-risk group, contributing to poor health outcomes.13 To address this challenge, our research aims to bridge critical knowledge gaps, develop and implement an integrated care package and provide evidence-based solutions to improve the health, quality of life and economic outcomes of vulnerable populations affected by these dual burdens. This approach leverages the organised and comprehensive nature of Pakistan’s TB programmes, offering multiple chances for diabetes intervention during the typical 6-month TB treatment period.
In this implementation science study, we will (1) design an integrated optimised tuberculosis-diabetes care package (Opt-TBD) and its implementation strategies; (2) pilot and refine the implementation strategies and (3) implement and evaluate the Opt-TBD care package in 15 TB care facilities of Pakistan.
Methods and analysis
Study settings
We will work with the TB programme across two provinces of Pakistan: Khyber Pakhtunkhwa and Punjab. TB care facilities (the basic management units, BMUs) will be selected from urban and rural settings of these provinces and will include three levels: primary, secondary (set up within secondary care hospitals) and tertiary care settings (within tertiary care hospitals).
Study design
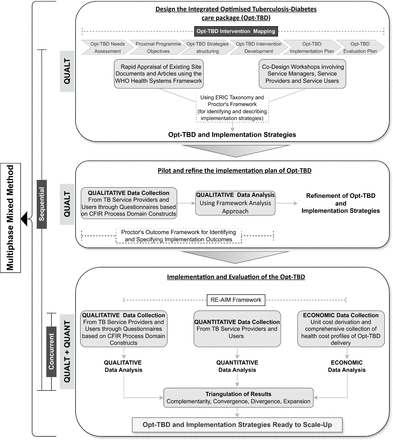
Our study will use a multiphase-sequential design across all three phases while phase 3 will predominantly employ a concurrent mixed-method design (QUAL→QUAL→QUANT+QUAL). In phase 1, we will use intervention mapping framework to develop the Opt-TBD care pathway and its implementation strategies using qualitative methods which will include (1) a rapid appraisal of the existing documents and (2) co-design workshops. Once designed, the acceptability and feasibility of the Opt-TBD care package and the implementation strategies will be determined qualitatively in phase 2. Finally, phase 3 will use concurrent qualitative and quantitative methods to evaluate the implementation strategies across 15 TB care facilities. Qualitative and quantitative methods will be given equal weight in analyses and interpretation through triangulation, aiming to get elaboration, enhancement and clarification of the results (ie, complementarity), identifying if the qualitative and quantitative data provide the same answer to the same question (ie, convergence or divergence) and gain breadth and depth of understanding (ie, expansion). Our study design is schematised in figure 1.
A multiphase sequential study design for designing, refining and implementing an optimised TB-diabetes care package (Opt-TBD).
An established set of frameworks will guide our work (table 1).
Application of implementation science frameworks across Opt-TBD implementation research phases
Supplemental material
Designing the integrated Opt-TBD care pathway and implementation strategies
We will use intervention mapping framework, comprising six steps: needs assessment, proximal programme objectives, strategy structuring, intervention development, implementation plan and evaluation plan, to formulate the Opt-TBD care pathway and associated implementation strategies. We will employ qualitative methods to conduct a rapid appraisal of documents and literature and co-design workshops, gathering and synthesising information essential for intervention mapping.
We have identified provisional components of the Opt-TBD based on WHO Guidelines11 and American Diabetes Association recommendations.19 We will begin with a rapid appraisal of the existing site documents and guidelines for further refining the components of the Opt-TBD. Findings of the rapid appraisal will be organised using the WHO Health Systems Framework.20 The Hermeneutic Approach21 will be applied in a five-step process, including gaining access to documents and data, validating document authenticity, comprehending documents, analysing data and extracting thematic information. This will be followed by co-design workshops, where TB and diabetes experts, healthcare staff, patients/family members and researchers will discuss and identify barriers and facilitators of local care systems. Findings from these workshops are expected to inform the subsequent development and implementation of the Opt-TBD intervention.
Following this, the Opt-TBD care package, incorporating the integrated care pathway and pertinent materials, will be developed. Stakeholders are expected to offer valuable feedback during co-design workshops to shape the care pathway and materials. The final pathway and materials will then be shared with all stakeholders for ultimate approval.
The next step, Opt-TBD Strategies identification, will adhere to the Expert Recommendations for Implementing Change (ERIC) taxonomy.22 Implementation strategies will be selected based on their alignment with barriers and facilitators identified in the previous steps. In this process, we will incorporate collaborative input from TB programme administration, facility staff and patients/caregivers. We will describe and operationalise selected implementation strategies, using Proctor’s framework.23 These strategies will be integrated into the Opt-TBD care pathway to develop the comprehensive Opt-TBD care package (referred to as Opt-TBD from this point onwards).
Finally, an evaluation plan will be developed to assess the feasibility, reach, effectiveness, adoption, implementation and maintenance of the Opt-TBD. The plan will emphasise the significance of a mixed-methods approach for a comprehensive evaluation, combining quantitative and qualitative methods to confirm relationships between implementation strategies and implementation outcomes while exploring contextual factors influencing these relationships.
Pilot and refine the implementation plan of Opt-TBD
At least three BMUs (primary, secondary and tertiary settings) will be purposely selected to pilot the Opt-TBD. In the selected BMUs, all newly diagnosed (within the last 4 weeks) adult patients with TB will be screened for diabetes. 18 patients per facility, confirmed as new diabetes cases, will receive Opt-TBD.
The total duration of this phase will be 8 months. During a couple of iterative cycles of piloting and refining, we will allow a month to embed the Opt-TBD into routine care and will assess its feasibility and acceptability at the third and sixth month follow-up of the patients with TB. The ‘Process domain’ of the Consolidated Framework for Implementation Research24 will guide the qualitative assessment of the Opt-TBD implementation strategies, while the Proctor’s Outcome Framework23 will be used to identify and specify the implementation outcomes for each implementation strategy.
With informed consent, we will conduct semistructured interviews with service managers and clinical staff (n=6–8) and patients (n=6–8) with TB and diabetes at two time points (third and sixth month) of the implementation period. Interview responses will be recorded and transcribed. Data will be analysed using framework analysis to capture the care package as delivered, understand its acceptability to patients and staff and explore contextual factors for success or failure. The most and least feasible and acceptable care package components and implementation strategies will be identified. Variations in between-site protocol adherence will be interpreted through contextual and implementation data. Using this information, we will refine the implementation strategies for delivering Opt-TBD.
Implementation and evaluation of Opt-TBD
The implementation
We will implement and evaluate Opt-TBD over 18 months in 15 purposefully selected TB care facilities across the provinces of Khyber Pakhtunkhwa and Punjab in Pakistan. These facilities will represent a mix of primary, secondary and tertiary care settings to ensure the intervention’s adaptability to diverse healthcare contexts.
Eligibility criteria for implementation study
Inclusion criteria:
Adults (≥18 years).
Newly diagnosed with active pulmonary drug-sensitive TB within the past 4 weeks, confirmed through sputum smear microscopy and molecular diagnostic methods (such as Xpert MTB/RIF).
Confirmed diagnosis of (pre)diabetes (newly diagnosed or existing) as determined through Random Blood Glucose and Glycated Haemoglobin.
Exclusion criteria:
Severe or unstable medical conditions that might hinder study participation, as determined by the Medical Officer at BMU.
Pregnancy or active breastfeeding.
Existing severe mental health conditions.
Identification and recruitment
Eligible patients at selected facilities will be offered diabetes screening and management by trained study staff who will explain the study’s objectives, procedures, potential benefits and potential risks. Written informed consent will be obtained from all participants. The consent form will be available in local languages and will include provisions for participants with limited literacy. Participants will be informed of their right to withdraw from the study at any point.
Over a 6-month period, an average of 80 patients per facility (1200 patients across all facilities) confirmed as having TB will be screened for (pre) diabetes and those with confirmed comorbid TB and (pre) diabetes will receive the Opt-TBD.
Intervention
The Opt-TBD will supplement existing TB care protocols outlined in the National TB Programme Guidelines25 with integrated (pre) diabetes care components based on collaborative care documents by The UNION26 and WHO.11 The package will likely contain advice on lifestyle modification, smoking cessation and reducing future risk of complications. A collaborative care pathway, developed during the first phase of the study, will define a clear referral mechanism for diabetes specialised care and coordinated follow-ups for both TB and diabetes. After the completion of TB treatment—usually at month 6—diabetes care will be transitioned to medical specialists/diabetologists based in participating hospitals. We will ensure this transition is seamless and agreed on during our stakeholder engagement co-design workshops.
The study will employ evidence-based implementation strategies, guided by the ERIC framework,22 to deliver the Opt-TBD care package. This includes capacity building initiatives for TB care providers, infrastructure changes to support integrated care, strategies to promote collaboration between TB and diabetes specialists, and the potential use of support and incentives to enhance healthcare provider engagement and patient follow-up.
The evaluation
We will assess the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM Framework)27 of Opt-TBD using concurrent mixed methods. We will first select which dimension(s) to evaluate and what assessments to conduct, ensuring relevance to the Pakistan context.
Quantitative data collection
In our study, we will extract data from two key groups: healthcare providers and recipients of the Opt-TBD care package. For TB-related outcomes, we will rely on routine data. Additionally, we will obtain informed consent from our study participants. Data collection about Reach, Effectiveness and Maintenance will focus on those who receive the care package. In contrast, data concerning Adoption, Implementation and Maintenance will be gathered from those responsible for delivering the care package. Our analysis will extend to assessing Reach across diverse categories, including gender, urban/rural settings, socioeconomic strata and Adoption across various staffing and organisational structures. We will scrutinise Fidelity indicators to gauge the degree of Implementation and Maintenance, and clinical outcomes will also be evaluated to measure Effectiveness. These measurements will be carried out at different time points: baseline, month 3, month 6 and month 12. Our baseline data will include demographic information, socioeconomic status and comorbid conditions. Descriptive statistics will summarise baseline data and carefully examine missing data patterns. If necessary, multiple imputations will be employed. Furthermore, we plan to conduct pre-specified subgroup analyses to explore potential differences based on gender, urban/rural context and socioeconomic factors. We will employ statistical techniques such as linear or logistic regression and mixed models to analyse all our outcomes.
Qualitative data collection
We will identify contextual factors impacting Reach, Effectiveness, Adoption, Implementation and Maintenance through in-depth interviews with healthcare providers, programme managers, stakeholders and Opt-TBD recipients. Topics will include the work environment, resources, beliefs in care package efficacy and patient illness burden. Approximately 40 interviews lasting 45–60 min will be conducted, transcribed, translated into English and analysed using framework analysis.
Economic analysis
The economic analysis will quantify the economic cost of delivering the Opt-TBD intervention from the healthcare system and patient perspective. We will capture resource use and costs, including staff, medications, devices, overheads and other healthcare resources directly from the healthcare provider. Unit cost data will come from partners and hospitals. Patient costs associated with the Opt-TB intervention will be collected at baseline and each follow-up via self-reported questionnaires. These costs include travel costs, time costs and any out-of-pocket healthcare and medication costs. We derive unit costs of providing Opt-TB and collect monetary data to present comprehensive health cost profiles from both the healthcare system’s and participants’ perspectives. The unit costs derived from the study will be scaled up to present budgetary costs for wider delivery of the intervention. Online supplemental table 1 outlines the assessments to be conducted.
Triangulation of findings
We will employ both qualitative and quantitative methods (as described in online supplemental table 1) concurrently, assigning equal importance to both in analyses and interpretation through triangulation. The goal is to elaborate, enhance and clarify the results by seeking complementarity between the two approaches. Additionally, we will investigate whether the qualitative and quantitative data yield consistent findings for the same research questions, exploring convergence or divergence. This approach aims to broaden and deepen our understanding of the Reach, Effectiveness, Adoption, Implementation and Maintenance of the Opt-TBD care package.
Patient and public involvement
Patients and the public were not involved in the designing/writing of this protocol. However, extensive participatory methods that involve both the patients and the public will be used during all the phases to design and evaluate the Opt-TBD care package.
Ethics and dissemination
Ethics approval for this study has been obtained from the National Bioethics Committee of Pakistan (reference no.: NBCR-1010). Written informed consent will be taken from all study participants.
We will produce and disseminate a TB-diabetes care package that includes clinical guidelines, a desk-guide and a training manual for healthcare workers. Additionally, we will conduct ‘train the trainer’ sessions to support the widespread adoption of the care package. These materials will enable health professionals to effectively manage comorbid TB and diabetes, ultimately enhancing patient care. National and provincial stakeholders will be engaged through strategic meetings, training activities and dissemination events. A TB-diabetes operational guide will be developed to support collaboration between TB and NCD programmes at various levels. The guide will include performance indicators to monitor service integration, ensuring that stakeholders have clear tools for assessing progress. Additionally, dissemination through academic publications, conferences and open-science platforms will ensure that the findings reach the global academic community. Community advisory panels will play a key role in ensuring patient engagement, helping to develop educational materials and a newsletter for research participants, while public awareness will be raised through social media, blogs and video content.
Discussion
Our study aims to address critical knowledge gaps in integrated TB and diabetes care. Specifically, we seek to enhance diabetes prevention and care for individuals with TB and explore effective strategies for organising care for individuals with TB and diabetes comorbidity. Additionally, our study will contribute to a broader understanding of how to tackle the challenge of multimorbidity, design integrated care across various health programmes and provide patient-centred care while potentially reducing healthcare costs. The bi-directional association between TB and diabetes is well-established, with diabetes significantly increasing the risk of TB and worsening treatment outcomes for patients with TB.28 29 In Pakistan, a country burdened by a high incidence of both TB and diabetes, this comorbidity poses a substantial public health challenge and places a significant economic burden on individuals, healthcare systems and the country as a whole.28–30 This often leads to changes in employment status or income due to health conditions.
Our research aims not only to enhance the prevention, diagnosis and management of diabetes in patients with TB but also to identify undiagnosed diabetes cases. This is crucial because both TB and diabetes can lead to adverse socioeconomic outcomes, exacerbating health disparities and poverty. Strengthening intersectoral collaboration and integrated care will also enhance data collection, leading to better-informed policy decisions and improved healthcare delivery in Pakistan.
The economic analysis of our proposed Opt-TBD will provide insights into the costs of scaling up integrated communicable and non-communicable disease care programmes. This can potentially reduce disease burdens and strengthen healthcare systems, a critical step toward achieving universal health coverage.
In conclusion, our research proposal holds significant promise in addressing the intertwined challenges of TB and diabetes in Pakistan, with potential implications for global health. It underscores the importance of integrated care, research capacity building and stakeholder engagement in advancing healthcare and socioeconomic development. If the effective implementation of the Opt-TBD is demonstrated, the unmet needs of more than 200 000 people with TB, diabetes and pre-diabetes in Pakistan could potentially benefit from improved clinical outcomes and quality of life every year. Health systems in Pakistan and globally will also benefit from this world-leading research on integrating and coordinating care across programmes and services, as well as offering services more efficiently using a patient-centred approach. There will also be potential to reduce the economic costs of multimorbidity and secure financial gains through cost savings and increased productivity.
Ethics statements
Patient consent for publication
Acknowledgments
The authors would like to acknowledge the support and collaboration of the National TB Control Programme Pakistan and Provincial TB Control Programmes Khyber Pakhtunkhwa and Punjab in the development and implementation of this study.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @asima khan
Contributors SAf and KS are the primary investigators who conceptualised and designed the study, obtained funding and are responsible for the overall conduct of the study. They jointly drafted the initial protocol manuscript and critically reviewed and revised the manuscript. Z, SAl, FKQ, SFJ and MS contributed to writing different sections of the proposal. ZK, AKN, NSa, AB and RI provided input into the study design. ZU-H, NSi, SP, RF and CH are senior advisors who provided guidance on the study design, analysis and overall implementation. All authors read, revised and approved the final manuscript. SA serves as guarantor and accepts full responsibility for the work and/or the conduct of the study and controlled the decision to publish.
Funding This study is funded under the Grand Challenge Fund which is supported by the World Bank and implemented by the Higher Education Commission of Pakistan (GCF-HEDP), reference number 20-GCF-770/RGM/R&ID/HEC/2021. This study successfully secured funding following a rigorous three-stage competitive review process.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; internally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.