Article Text
Abstract
Introduction Orofacial clefts (OFCs), including cleft lip, cleft palate and combined cleft lip and palate, are among the most common craniofacial malformations in newborns and present significant healthcare challenges. Emerging evidence has raised concerns regarding the potential impact of prenatal exposure to antibiotics on fetal development. Antibiotics prescribed during pregnancy—particularly those that cross the placental barrier—may pose teratogenic risks. Previous studies investigating the association between prenatal antibiotic exposure and the risk of OFCs have yielded inconsistent results. However, no studies have yet attempted to summarise this evidence, highlighting the need for a comprehensive evaluation. This report describes a systematic review and meta-analysis protocol to retrospectively analyse the relationship between prenatal antibiotic exposure and the risk of developing OFCs, focusing on the role of antibiotic type and timing of exposure. The results of such a review will hopefully provide a comprehensive synthesis of the available evidence, helping to inform clinical practice and guide patient counselling regarding the use of antibiotics during pregnancy.
Methods and analysis The planned systematic review and meta-analysis will adhere to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines to ensure a comprehensive and systematic approach to summarising the available evidence on the topic. This study will include longitudinal cohort studies, case–control studies, and interventional trials that investigate the association between prenatal antibiotic exposure and OFCs. The search strategy will cover major databases, including CINAHL, Cochrane Library, ClinicalTrials.gov, EMBASE, PubMed, Scopus and Web of Science, using tailored search terms. A team of independent assessors will screen article titles, abstracts and full texts. Any discrepancies will be resolved through discussions. Quality assessment will use the Newcastle-Ottawa Scale and Grading of Recommendations Assessment, Development and Evaluation criteria. Data extraction will focus on the study characteristics, participant details, exposure specifics and outcome measures. A random-effects meta-analysis will aggregate summary effect sizes, and heterogeneity will be assessed using I2 and Q statistics.
Ethics and dissemination Ethical approval is not required for this systematic review, as it relies on already published data. The findings will be disseminated through peer-reviewed journals and conference presentations, providing critical insights into clinical practice and public health policies regarding antibiotic use during pregnancy.
PROSPERO registration number CRD42024565064
- Anti-Bacterial Agents
- Fetal medicine
- EPIDEMIOLOGY
- Child
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
A comprehensive search strategy across multiple databases and grey literature sources will be employed while adhering to Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines, ensuring thorough identification of relevant studies.
Study selection, data extraction and quality assessments will be performed by two independent reviewers, enhancing accuracy and consistency.
The use of established quality assessment tools, including the Newcastle-Ottawa Scale and Grading of Recommendations Assessment, Development and Evaluation criteria, will improve the reliability and validity of the findings.
Variability in study designs, populations, antibiotic types and timings of exposure may introduce heterogeneity, complicating the synthesis of results and resulting in publication bias.
Introduction
Orofacial clefts (OFCs) are among the most common craniofacial malformations in newborns and present significant healthcare challenges because of their complex aetiology and multifaceted impact on health.1–3 They affect an estimated 4.6 million individuals globally, resulting in a burden of ~529 758.92 disability-adjusted life-years.4 OFCs—which include cleft lip (CL), cleft palate (CP) and combined CL and palate (CLP)—result from disruptions in the normal development of the orofacial region during embryogenesis2 and are specifically influenced by a combination of genetic, environmental and maternal lifestyle factors.2 3 5 Environmental influences, including maternal smoking, alcohol consumption, nutritional deficiencies and medication use during pregnancy, have been implicated in the pathogenesis of OFCs.5–7
Recent research has raised concerns regarding the potential impact of prenatal exposure to antibiotics on fetal development.8–10 Antibiotics are commonly prescribed during pregnancy to manage infections that, if left untreated, can pose significant risks to both the mother and the developing fetus.11–13 However, the teratogenic potential of antibiotics, particularly those that cross the placental barrier, remains a topic of considerable debate and investigation.14 Potential mechanisms through which antibiotics may contribute to the development of OFCs include the disruption of normal cellular processes, interference with folate metabolism and induction of oxidative stress in the developing embryo.15
Several studies have suggested an association between prenatal antibiotic exposure and an increased risk of OFCs.16–19 However, current evidence on the matter is inconsistent overall,20–23 varying significantly across different antibiotic classes, dosages and timings of exposure. Specifically, the current body of literature on this topic is characterised by heterogeneity in study designs and populations, potentially due to variations in study populations, classifications of antibiotic exposure and the control of confounding variables. Therefore, a systematic approach to synthesising this evidence is essential for drawing robust and generalisable conclusions. Understanding the potential teratogenic effects of antibiotics is crucial for clinical practice and public health. Therefore, the following proposed study is not only important for informing clinical practice and guiding patient counselling but also pivotal for shaping public health policies and future research trajectories in prenatal care and teratology.
Objectives
The objective is to answer the following PICO (Population, Intervention, Comparison, Outcome) question: what is the association between prenatal antibiotic exposure and the risk of developing OFCs in children, compared with the risk in children with no antibiotic exposure during pregnancy?
Specific objectives:
To systematically review studies investigating the relationship between prenatal antibiotic exposure and the risk of developing OFCs, with a particular focus on elucidating the role of antibiotic type, dosage and timing of exposure.
To conduct a meta-analysis that quantitatively synthesises data from individual studies, providing a robust estimate of the association between prenatal antibiotic exposure and the risk of OFCs while accounting for potential moderating factors.
Methods and analysis
Protocol development
The protocol for this study aligns with the guidelines established by the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P).24 25 The PRISMA-P checklist is provided in online supplemental file 1. The registration code for this review protocol (CRD42024565064) will be accessible on the International Prospective Register of Systematic Reviews (PROSPERO). Any updates to the protocol and review process will be promptly reflected in the PROSPERO registration. The study is scheduled to commence in December 2024 and is expected to be completed by May 2025.
Supplemental material
Eligibility
PICO framework
Population: pregnant individuals and their children (mother–child pairs).
Intervention/exposure: prenatal exposure to antibiotics.
Comparator: no exposure to antibiotics.
Outcome: the occurrence of OFCs, CL, CP and CLP.
Inclusion and exclusion criteria
Participants
The participants in this study will be mother–child pairs. We will include all pregnant individuals regardless of age, ethnicity or health status. We will include studies with explicit documentation of antibiotic exposure during pregnancy—including prescription records, patient self-reports and medical notes confirming antibiotic use. The exclusion criteria include studies lacking specific information regarding the pregnancy status of the participants or those with ambiguous details regarding the timing and confirmation of pregnancy. We will also exclude studies in which antibiotic exposure is not explicitly linked to the pregnancy period or where such exposure is inferred but not documented.
Exposure
Exposure to any class of antibiotics during pregnancy—including (but not limited to) penicillins, cephalosporins, macrolides, tetracyclines, fluoroquinolones and sulfonamides—will be included. We will analyse antibiotic exposure by individual classes to better understand the potential differences in risks associated with each class. Given that certain classes of antibiotics are contraindicated during pregnancy,12 this approach will allow for a more detailed analysis of class-specific effects. We will include studies that provide details regarding the type of antibiotic (specific name or class), frequency of intake and trimester during which the exposure occurred. The primary focus will be on exposures occurring during the first trimester, as this is the period when OFC development occurs.26 This detailed information is crucial for understanding the potential dose–response relationships and most critical periods of fetal development. Studies with grouped antibiotic exposures that do not distinguish between different antibiotics or classes will be excluded. This is due to the fact that different antibiotic classes may have distinct risks and mechanisms of action, and combining them may mask the specific effects associated with each individual class. Reports with unclear or inconsistent information regarding the frequency, dosage or timing of antibiotic exposure during pregnancy will also be excluded.
Comparator/control
Groups of pregnant individuals who did not receive any antibiotics during pregnancy will serve as a baseline control group for comparisons. In addition to the baseline control group, we will include active comparators, such as pregnant individuals who were prescribed antibiotics known to be safe during pregnancy. This will help account for potential confounding by indication, as some bacterial infections themselves may increase the risk of birth defects.27 Furthermore, we will consider comparator groups based on the timing of antibiotic exposure (eg, first-trimester exposure vs second and/or third-trimester exposure). Finally, we will exclude studies that lack a well-defined control or comparison group, thus failing to provide a clear contrast for the analysis.
Outcomes
The primary focus of this study will be on OFCs, including both syndromic and non-syndromic CL, CP and combined CLP. All OFC diagnoses should be based on clinical examinations, medical records or standardised screening protocols—either prenatally, at birth or within a defined postnatal period (up to 1 year). Studies that focused on outcomes unrelated to OFCs or speculative studies will be excluded.
Types of studies
Longitudinal cohort studies (both prospective and retrospective), case–control studies and interventional trials that quantitatively assessed the relationship between prenatal antibiotic exposure and OFCs will be included. All included studies must have a sound methodological design, including well-defined populations, clear exposure and outcome measures, and appropriate statistical analyses. Cross-sectional studies will be excluded as they do not establish a temporal relationship between exposure and outcome; therefore, causality cannot be inferred. We will also exclude case reports and case series, as they often lack generalisability, as well as reviews, editorials, commentaries and animal studies because they typically do not provide original empirical data. Studies with methodological flaws, such as inadequate sample sizes or lack of statistical rigour, will also be excluded. No restrictions will be applied regarding the language of the studies or publication length, and translation will be performed using Google Translate or by individuals capable of providing a translation.
Search strategy and study selection
Our search methodology has been meticulously designed to identify both published and unpublished studies. This comprehensive approach covers electronic repositories, conference records, virtual platforms, scholarly dissertations and (if necessary) direct correspondences with primary authors. We will search databases including CINAHL, Cochrane Library, ClinicalTrials.gov, EMBASE, PubMed, Scopus and Web of Science. Index terms and keywords will be carefully tailored to the unique characteristics of each database. Google Scholar will also be used to search for grey literature and ongoing studies. The following search terms will be combined and adapted as needed to meet the database specifications: (“antibiotics” OR “antimicrobial agents” OR “anti-infective agents” OR “antibacterial drugs” OR “broad spectrum antibiotics” AND “prenatal exposure delayed effects” OR “maternal exposure” OR “intrauterine exposure” OR “gestational drug exposure”) AND (“orofacial clefts” OR “cleft lip” OR “cleft palate” OR “congenital defects” OR “birth defects” OR “congenital anomalies” AND (“observational study” OR “longitudinal study” OR “retrospective study” OR “prospective study” OR “epidemiological research”). Both MeSH terms and other appropriate subject headings will be used in the database search. The search strategy for the databases is illustrated in online supplemental file 1.
A team of three independent assessors will conduct the search. The retrieved studies will be imported into Rayyan (https://rayyan.ai/), a systematic review software platform28 Initially, two independent assessors will assess the suitability of the titles and abstracts of all screened publications, followed by full-text examinations in accordance with our inclusion criteria. Two assessors will concurrently evaluate the abstracts and full-text materials, and any discrepancies will be resolved through comprehensive discussions. Figure 1 illustrates the screening process. In cases where significant disparities persist, the ultimate resolution will be entrusted to the other assessors.
Flow chart illustrating the study process, following the PRISMA-P guidelines. PRISMA-P, Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols.
Quality assessment
Two assessors will independently evaluate the methodological rigour of the included studies using the Newcastle-Ottawa Scale to assess the quality of the non-randomised studies included in the meta-analyses. If randomized controlled trials (RCTs) are included, the Cochrane Risk of Bias (ROB V.2)29 tool will be applied. Additionally, two assessors will independently apply the Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessment to categorise the quality and strength of evidence in each publication as high, moderate, low or very low in terms of its primary outcomes. Any discrepancies between assessments will be resolved through constructive discussions. The assessment of methodological quality will be integrated into the discussion of the respective study findings.
Data extraction
Two assessors will independently review the included studies and extract relevant information using a predesigned form. Discrepancies will be resolved through discussion until a consensus is reached. The data extracted from each study will encompass, though not be limited to (a) study characteristics: author names, publication year, citation, study location, study design, study dates, participant selection criteria, statistical analysis methods, funding sources and conflicts of interest; (b) participant characteristics: including the number of mother–child pairs (sample size), sampling methods, sex, age and any reported sociodemographic data; (c) exposure: type of antibiotic (specific name or class), frequency of use, the trimester during which exposure occurred and the method of data collection; (d) comparator: participants who were not exposed to antibiotics; (e) outcome: type of OFCs, such as CL, CP or combined CLP, the method of outcome data collection, and covariate adjustment in the analysis and (f) results: statistical outcomes, including effect measures, confidence intervals and p values.
OFC diagnoses must be confirmed through clinical examinations, medical records or standardised screening protocols, either during the prenatal stage, at birth or within a specified postnatal period. Secondary outcomes may include variations in the risk of OFCs based on antibiotic dosage, active comparators and socioeconomic status. We will prioritise studies with the clearest and most consistent definitions of both exposure and outcomes.
Data analysis and synthesis
The findings will be reported and presented in accordance with the PRISMA statement.30 Additionally, if only observational studies are included, we will also follow the Meta-Analysis of Observational Studies in Epidemiology checklist.31 The findings will be comprehensively presented using tables and figures. The effect size (ES) of interest will be the relative risk (RR). When effect estimates are provided as crude or adjusted ORs or RRs, we will prioritise collecting the adjusted estimates and provide a descriptive summary of the covariates adjusted for each study. In cases where the ES is not present, an unadjusted RR will be estimated from contingency tables. ORs will be converted to RRs using appropriate formulae. Both fixed-effect and random-effects meta-analyses will be used to estimate the pooled ESs along with their associated 95% CIs. Significance levels will be documented. Interstudy variability, measured using the Cochrane Q or  statistics, will be explored, as well as potential impacts from smaller studies.
statistics, will be explored, as well as potential impacts from smaller studies.  values of 25%, 50% and 75% will be assumed to represent low, moderate and high heterogeneity, respectively. The significance of heterogeneity will be determined via
values of 25%, 50% and 75% will be assumed to represent low, moderate and high heterogeneity, respectively. The significance of heterogeneity will be determined via 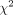 values for Q statistics, with p<0.05. If the level of between-study heterogeneity is higher (
values for Q statistics, with p<0.05. If the level of between-study heterogeneity is higher ( >75%), a random-effects meta-regression will be performed to identify the potential moderators. A leave-one-sample-out validation will be used to explore the influences of each included study on the pooled ES. Funnel plots will be used to detect potential publication bias and small-study effects. A p<0.05 will be considered indicative of statistically significant publication bias. For outcomes with more than 10 individual studies, we will use Egger’s regression test to assess asymmetry. The combined ES will be visually represented using a forest plot. If the number of studies included is insufficient (<3), a narrative review of the study findings will be presented instead of a meta-analysis.
>75%), a random-effects meta-regression will be performed to identify the potential moderators. A leave-one-sample-out validation will be used to explore the influences of each included study on the pooled ES. Funnel plots will be used to detect potential publication bias and small-study effects. A p<0.05 will be considered indicative of statistically significant publication bias. For outcomes with more than 10 individual studies, we will use Egger’s regression test to assess asymmetry. The combined ES will be visually represented using a forest plot. If the number of studies included is insufficient (<3), a narrative review of the study findings will be presented instead of a meta-analysis.
Sensitivity analyses on primary outcomes will be conducted and will involve excluding studies with a high risk of bias or incomplete data. Efforts will be made to contact researchers or study sponsors to obtain any missing information. If this is not possible, established methods for estimating missing data using multiple imputations will be applied. The validity of imputed data will be assessed through a sensitivity analysis. Subgroup analyses will explore the effects of medication dosages and active comparators. Furthermore, we will conduct a meta-regression analysis across various subgroups, including, but not limited to, confounding factors (crude or adjusted), socioeconomic status and study design. All statistical analyses will be conducted in Stata V.18 (StataCorp).
Discussion
This systematic review and meta-analysis protocol represents the first comprehensive effort to investigate the association between prenatal antibiotic exposure and the risk of OFCs. Its findings are expected to provide critical insights into medication safety during pregnancy and its potential teratogenic effects on fetal development, particularly regarding the occurrence of OFCs. Our conclusions will be based on the combined results of the included studies, presented through quantitative analysis or narrative synthesis.
Understanding the relationship between prenatal antibiotic exposure and OFCs is crucial for clinical decision-making and patient counselling. If a significant association is found, it will highlight the need for careful consideration of antibiotic prescriptions during pregnancy. This could lead to the development of guidelines and protocols aimed at minimising unnecessary antibiotic use and selecting safer alternatives when treatment is necessary. The results will also hopefully ensure that healthcare providers are better equipped to inform expectant mothers about the potential risks and benefits of antibiotic use during pregnancy, thereby facilitating more informed choices.
The major strengths of this planned, systematic review lie in its rigorous methodological approach, adherence to the PRISMA-P guidelines and use of established quality assessment tools such as the Newcastle-Ottawa Scale and GRADE criteria. However, several methodological challenges must also be addressed. First, the included studies are likely to vary in their designs, populations, antibiotic types, dosages and timing of exposure, which may introduce significant heterogeneity. This variability can complicate the synthesis of the results and limit the generalisability of our findings. Subgroup and sensitivity analyses will be crucial to addressing these issues. Second, the potential under-representation of unpublished or negative findings may also introduce publication bias. Funnel plots and Egger’s tests will be used to assess and mitigate this bias; however, its presence cannot be entirely ruled out. Third, the conclusions drawn from this review will depend on the methodological quality and reporting standards of the included studies. Variations regarding how antibiotic exposure and OFCs are defined and measured may impact the robustness of the findings. The use of the GRADE criteria will help evaluate the strength and quality of the evidence, providing a clearer understanding of the confidence that can be placed in the results. Nevertheless, this review is expected to identify gaps in the current literature and suggest areas for future research.
Ethics and dissemination
Obtaining ethical approval was not deemed necessary for this study, as it pertains to a protocol for a systematic review that relies on published, and therefore, publicly available data. The findings of this study will be communicated through peer-reviewed articles and presentations at conferences.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @AbNagata, @issey0024
Contributors AN, MSR and MMR conceived of the study’s concept and drafted the initial version. TK, SS, KO and MR provided guidance to the research teams. All of the authors participated in drafting and revising the manuscript, formulating the review questions and designing the study. All of the authors have read and approved the final version of this manuscript. AN serves as the guarantor, accepting full responsibility for the work and/or the conduct of the study, has access to the data and controls the decision to publish.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.



