Article Text
Abstract
Introduction Cerebral palsy (CP) presents a complex neurodevelopmental disorder with a spectrum of motor impairments stemming from early brain injury. Whereas CP is traditionally viewed as a non-progressive condition, emerging evidence suggests a progressive decline in mobility and function, particularly in adulthood. Despite the prevalence of self-reported age-related gait decline in adults with CP, objective evidence supporting this phenomenon remains limited. Moreover, mechanistic insights into these functional alterations and their comparison with typically developing (TD) peers are lacking. To address this gap, our study aims to objectively assess age-related changes in gait performance among individuals with CP while examining physiological differences compared with TD peers.
Methods and analysis This protocol will compare the mobility of individuals with and without CP within two age groups (18–25 and 35–50 years old). Participants at Gross Motor Function Classification System levels I–II at age 18 will be invited to partake in the study. Every participant will be invited to complete four visits investigating a wide range of mobility related measures: walking performance, muscle strength, cardiopulmonary performance, fatigability, cost of walking and quantitative gait analysis. Through this comprehensive analysis encompassing gait performance metrics, self-reported outcomes, muscle strength, biomechanics and metabolical cost of walking, and fatigability, we seek to elucidate the underlying mechanisms driving age-related gait decline in adults with CP and inform targeted interventions to maintain function and quality of life.
Ethics and dissemination The study has been approved by the French ethics board (#2022-A02510-43) and will be communicated through conferences, articles and to participants through layman terms.
Trial registration number NCT06163950.
- Adult neurology
- Neuromuscular disease
- Fatigue
- Gait Analysis
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
A comparison will be made between two age groups within individuals with cerebral palsy (CP) (ie, young and older), those groups being further compared with groups of matched healthy volunteers, allowing for a thorough investigation of the effects of ageing in adults with CP.
This study comprises multiple gait-related measures offering an in-depth investigation of potential pathophysiological mechanisms of gait decline due to ageing with CP.
This study employs a novel and ecological test of fatigability, not previously used in the population with CP, but validated among healthy peers and other clinical populations.
The calculated sample size limits certainty pertaining to secondary outcomes, but allows estimation of age-related differences.
The patient group is heterogenous with some participants needing assistive devices during testing.
Introduction
Cerebral palsy (CP) is the most prevalent childhood onset disability, arising from an insult to the developing brain. CP manifests in a spectrum of forms, primarily distinguished by the affected limbs (uni- or bilateral) and motor type (spastic, dyskinetic, ataxic), with bilateral spastic CP being the most common presentation.1 Alongside this heterogeneity, CP is associated with a range of issues, including cognitive impairments2 and decreased motor control3 4 as main CP-related issues, as well as musculoskeletal morbidities,5 6 pain7 and/or fatigue8 9 as secondary issues, alongside co-existing issues such as comorbid diseases.10–13 Although CP is considered non-progressive, the consensus among clinicians, researchers and patients suggests that mobility issues tend to progress over life.14 This progression often manifests as a decline in mobility during the third and fourth decades of life, as evidenced by numerous cohort, questionnaire and interview studies.2 15–21 However, whereas self-reported age-related decline in gait performance in adults with CP is widely acknowledged, objective evidence supporting this alteration remains scarce.
There is also a paucity of studies proposing mechanistic hypotheses to elucidate these functional alterations. Although comparisons between individuals with CP to the typically developing (TD) population are common, studies focusing on the ageing process within this context are notably lacking, except for those examining musculoskeletal morbidities and comorbid diagnoses, which tend to worsen with age.5 6 13 22–24 In the TD population, gait performance typically declines with age due to a combination of factors, including decreased muscle strength, increased metabolical cost of walking associated with changes in gait biomechanics and increased exercise-induced fatigability.25–28 It is therefore plausible to speculate that such ageing-induced alterations are exacerbated in individuals with CP.15 20 Indeed, ageing appears to cause alterations to the neuromuscular3 and cardiovascular29 30 systems at an earlier age in adults with CP compared with their TD peers, alongside alterations in the biomechanics of walking.31–33 Further studies are however needed to comprehensively understand age-related gait decline in individuals with CP, with the potential to inform strategies for maintaining their function and QoL.
The aim of the current investigation is therefore to objectively assess the decline in gait performance with ageing in individuals with CP while also examining clues regarding how their physiological particularities change compared with TD peers as they age. To achieve this objective, we will investigate differences in gait performance, self-reported outcomes, muscle strength, metabolical cost and quantitative gait analysis (QGA) of walking, and fatigability between young (18–25 years old) and older (35–50 years old) groups of individuals with CP and TD peers. In the present study, our primary objective is specifically to compare exercise-induced fatigability (ie, the decline in force production capacities after a standardised fatiguing task) among young and older CP and TD groups. Our secondary objectives are to compare all the other aforementioned parameters between groups.
Methods and analysis
Design of the study
The study, approved by the French Ethical board (approval #2022-A02510-43), commenced data acquisition in June 2023 and is scheduled to conclude in September 2024. It is registered on ClinicalTrials.gov (registration # NCT06163950). It is an observational case-control study encompassing four experimental groups: young (18–25 years old) and older (35–50 years old) individuals with CP and corresponding healthy individuals, with matching based on age, gender and body mass index (BMI). Participants will attend four sessions at our university laboratory, spaced at least 1 week apart (figure 1). During the initial visit, participants will undergo inclusion, medical evaluation, anthropometric measurements and completion of questionnaires, as well as clinical tests including a 6-min walking test with oxygen consumption recording and a cardiopulmonary exercise test (CPET). During this initial visit, participants will also be familiarised with dynamometry measurements, walking on the treadmill and the fatigability protocol. The second visit will involve the 6-min walk test without oxygen consumption recording, strength measurements of the knee extensors and 3D gait analysis (only for CP groups). During the third visit, participants will complete strength measurements of the plantar flexor and knee flexor muscles, followed by the measurements of oxygen consumption during walking on a treadmill at different speeds. The fourth visit will be dedicated to the evaluation of exercise-induced fatigability. The study did not include patient or public involvement. BF is a long-term clinician within the field and have provided information regarding typical patient complaints which have assisted in forming the methodology.
Study overview.
Participants
The study aims to recruit individuals with CP and TD peers aged 18–25 or 35–50 years. For participants with CP, inclusion criteria include diplegic spastic CP, ability to walk for 6 min continuously and Gross Motor Function Classification System I–II at age 18 (ie, as retrospectively assessed through medical records and patient reports). For TD counterparts, inclusion criteria involve being comparable to a participant in the CP group in terms of BMI, age and gender. Due to the expected challenges in finding suitable participants with CP, no matching will be attempted between the two age groups. Exclusion criteria for both groups consist of contraindications to maximal effort, recent lower limb surgery (ie, within 6 months), pregnancy and being under legal guardianship. Additionally, individuals with CP should not have received botulinum toxin injection within the last 3 months. Participants with CP will be sourced through medical treatment centres by their respective doctors who will be responsible for the first point contact and recruitment. Participants acting as TD controls will be sourced by posters and information spread by the university.
Sample size calculation
An a priori sample size calculation was conducted using G*power 3.1.34 Fatigability (ie, defined as the exercise-induced force loss) was chosen as the primary outcome measure due to its clinical relevance in understanding the functional limitations experienced by individuals with CP. The calculation was based on the hypothesis that there would be an age group × fatigability difference within participants with CP, as already observed in the TD population.28 Indeed, using the same fatigability protocol as proposed in the present study, Varesco et al,28 investigated age-related differences in exercise-induced fatigability between young (18–30 years old) and older (60–80 years old) healthy volunteers, and greater fatigability was reported for the older group with an effect size of 1.13. Assuming an exacerbated effect of age on individuals with CP,20 35 we expect in the present study a similar effect size between young (18–25 years old) and older (35–50 years old) individuals with CP. With an alpha (α) of 5% and a power of 90, the power calculation indicated a need for 18 participants per group.
Fatigability protocol
Exercise-induced fatigability will be investigated on a recumbent bike (figure 2), as in previous studies,28 36 37 with the aim to quantify the decline in voluntary force production during exercise and at exhaustion. The recumbent bike setup includes blockable force-measuring pedals (PowerForce pedal, Model PF1.0.0, Radlabor GmbH, Freiburg, Germany) to evaluate maximal voluntary force of the right leg. The fatiguing protocol will consist of body weight-dependent 3-min stages with resistance increasing incrementally (stages 1–5, + 0.3 W.kg−1; stages 6–10, + 0.4 W.kg−1; stages over 11, + 0.5 W.kg−1), until task failure. During the protocol, participants will be provided visual feedback on the cadence and verbal encouragement. Immediately (~2 s) after each stage end, the pedals will be blocked to allow for neuromuscular evaluation within a 1-min period before continuing to the next stage. Neuromuscular evaluation will be further performed at task failure, defined as being unable to maintain a cadence of more than 40 RPM. Neuromuscular evaluation will consist of a 4-s maximal voluntary contraction during which femoral nerve electrical stimulation will be superimposed (superimposed twitch (SIT)). Then, another stimulation will be delivered at rest (Twitch)~2 s after full relaxation. Visual feedback of the force signal and strong verbal encouragement will be provided during maximal efforts. Voluntary activation (VA) will be assessed according to the following formula38 39:

Illustration of the bike ergometer. Artwork by Giorgio Varesco.
To allow nerve electrical stimulation, single-pulse square-wave stimuli of 1-ms duration (maximal voltage: 400 V) will be delivered over the femoral nerve through a constant current stimulator (DS7A, Digitimer, Welwyn Garden City, Hertfordshire, UK). The cathode electrode (Kendall MediTrace) will be placed on the inguinal triangle, and the anode (50×90 mm Durastick Plus electrode; DJO Global, Vista, CA) placed midway between the great trochanter and the lower border of the iliac crest. The stimulation intensity will be progressively increased by 10 mA until force plateaus. To ensure that all motor axons will be spatially activated, this current intensity recorded at plateau will be increased by 20%. Moreover, throughout the protocol, participants will be equipped with electrodes for electromyography (EMG) measurements of the vastus lateralis muscle of the right leg. Data will be collected using LabChart software (AD Instruments, Bella Vista, Australia) and a combination of an amplifier (Octal bio amp ML138, AD Instruments, Bella Vista, Australia) and a PowerLab system (16/30-ML880/P or 16/35-PL3516, AD Instruments, Bella Vista, Australia).
The fatiguing protocol’s outcomes will include number of completed stages, EMG activity during stages, as well as data on maximal torque (N or N/kg), VA (%) and twitch amplitude (N or N/kg) across stages and at task failure. Decrease in maximal torque across stages (ie, fatigability) will be our primary outcome. Similar to the beforementioned studies, the data analysis will compare groups based on the latest common stage finished and at exhaustion.
Clinical tests
All clinical tests outlined below were designed to assess various aspects of physical function in participants with CP and their TD counterparts and to be performed without using walking aids (unless being necessary to complete the test).
10-metre walking test
On a flat surface in a corridor, participants will have to walk as fast as possible between two gates (WITTY·GATE, Microgate Srl, Bolzano Italy) situated 10 metres apart. Participants will be instructed to start walking 1 metre before the first gate and to finish 1 metre after the second one. Each trial will consist of four attempts, with the average of the two fastest trials used for analysis of time performance (s).
Timed-up-and-go
Participants will sit on a chair with armrest and will be tasked with standing up, walking to, around and returning to the seated position from a cone situated three metres away. Two trials will be filmed, and the fastest one will be used for analysis.
Six-minute walking test
Participants will be instructed to walk as far as possible in 6 min. The test is administered in a 30-metre straight flat corridor with cones at each end, performance being measured in distance (m). Verbal encouragement will be provided, and time remaining will be communicated every 2 min. Participants will wear their usual footwear. In one condition (ie, first visit), oxygen consumption will be measured online using a portable spirometer system (Metamax 3B, Cortex biophysik GmbH, Leipzig, Germany) to calculate the cost of walking (see the ‘Cost of walking’ part for more details on the calculation method). Data will be analysed using Metasoft Studio (Cortex biophysik GmbH, Leipzig, Germany). In the second condition (ie, second visit), participants will walk without the portable spirometer system.
Berg Balance Scale
The Berg Balance Scale is a 14-point scale assessing balance in adult populations.40 Test scores range from 0 to 56 with higher scores indicating better balance. All unilateral tests will be conducted on both limbs. Due to a ceiling effect, only individuals with CP will undergo this test.
Actigraphy
Participants will be asked to wear continuously throughout the day and night an accelerometer (wGT3X-BT, firmware 1.9.2, ActiGraph, Pensacola, FL, USA) on their non-dominant wrist for a week following the first visit. The accelerometer will be set to a 30 Hz sampling rate, all lights turned off and ‘idle sleep mode’ disabled. If it causes discomfort while sleeping, participants will be allowed to put it aside and equip it again as soon as possible in the morning. Data on activity levels (light, moderate and vigorous) (min), sleep duration (min), sleep efficiency and step count will be collected and analysed offline (ActiLife v6.13.4, Actigraph, Pensacola, FL, USA). Further analyses will be conducted using dedicated packages (GCIR, Verisense Step count and read.GTX) on R.41–44
Questionnaires
Participants will complete several questionnaires administered using LimeSurvey (LimeSurvey GmbH., Hamburg, Germany) in validated French versions. The investigator will be present during questionnaire completion but will not be able to see the screen to ensure participant privacy. Assistance will be provided if any questions are unclear.
Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F)
Fatigue over the last 7 days will be measured using the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire,45 a shortened version of the full scale FACIT questionnaire focusing on fatigue in patient populations. The FACIT-F scale consists of a 13-item, unidimensional measure of fatigue,45 with each item answered on a 5-point scale. The total score ranges from 0 to 52, with a lower score indicating more fatigue and below 34 being considered clinically significant fatigue in patients with cancer.46
Short Form 36 Health Survey Questionnaire (SF-36)
Quality of life (QoL) over the last 4 weeks will be assessed using the French version of the Short Form 36 Health Survey Questionnaire (ranges from 0 to 100 with higher scores indicating more favourable functional status).47 It is a self-report measure of functional health and well-being. The questionnaire includes physical and mental components, with the sum of their scores used to calculate total score for QoL.
Godin-Shephard Leisure-Time Physical Activity Questionnaire
Leisure-time exercise over a week will be assessed using the Godin-Shephard Leisure-Time Physical Activity Questionnaire.48 It assesses the frequency and duration of mild, moderate and strenuous exercise through four simple questions. The greater the composite score obtained, the more respondents are physically active.
Cardiopulmonary exercise test (CPET)
A maximal incremental cycling cardiopulmonary exercise test on a cycle ergometer. Participants will be installed on a bike ergometer (CP (Cortex bike M, Cortex biophysik GmbH, Leipzip Germany); TD (SRM SmartIT, SRM GmbH, Jülich Germany)) with gas exchange monitoring (Metamax 3B, Cortex biophysik GmbH, Leipzip Germany). The protocol will consist of progressive increases in load and will conclude when the participant fails to maintain more than 40 RPM. The power output at task failure will be considered maximum exercise capacity (Pmax). Participants will be strongly encouraged throughout the test. The initial resistance will be tailored to each participant, so will the progressive load increases (10–30 W/min), with the aim to conclude the test within 8–12 min to minimise the risk of neuromuscular or mental fatigue.49 50 Lactate measures (CP (Biosen C-Line Clinic, EKF Diagnostics, Cardiff, UK); TD (Lactate Scout 4, EKF Diagnostics, Cardiff, UK)) will be taken 3 min after volitional failure, with the 3 min consisting of 1 min of active recovery and 2 min of passive recovery. During the test, participants with CP will have their heart rhythm monitored (echocardiogram) to ensure that physical exertion is well tolerated. The highest rolling 30-s average for VO2 will be calculated to express V̇O2peak in both absolute values (L·min−1), and relative to body mass (mL·min−1·kg−1).
Quantitative gait Analysis
The QGA will be conducted only on participants with CP using advanced technology to comprehensively evaluate gait patterns. A 12-camera (Raptor-4 & Cortex software, Motion Analysis Corporation, Rohnert Park, CA, USA) will be used in conjunction with six force plates (90×90 cm, Model 9287C, Kistler, Winterthur, Switzerland) and wireless EMG electrodes (Trigno Wireless EMG System, Delsys, Natick, USA). Forty-six reflective markers will be placed on participants’ lower body using the Rizzoli lower body marker system51–53 and the Amsterdam Foot Model.54 55 After shaving, abrading and cleaning the skin, EMG electrodes will be placed on specific muscles: the biceps femoris (long head), vastus lateralis, rectus femoris, gastrocnemius lateralis and tibialis anterior according to Surface Electromyography for the Non-invasive Assessment of Muscles guidelines. Participants will be instructed to walk at their normal self-selected pace and without any assistive device along a 10 m designated path while wearing markers and electrodes, allowing gait analysis to capture data on kinematics, kinetics and EMG. This will provide a comprehensive assessment of gait biomechanics in individuals with CP. Clinical practice of this test has indicated that approximately 15 walking trials are sufficient to gather adequate data on the entire gait cycle. Data obtained from the QGA will be processed to generate a Gait Profile Score,56 which provides a summary measure of gait quality.
Strength measurements
All strength measurements will be conducted using an isokinetic dynamometer (Con-Trex MJ, Physiomed Elektromedizin AG, Schnaittach, Germany). Participants with CP will undergo testing on their most affected leg, while TD participants will have their non-dominant leg tested. Prior to testing, participants will engage in a warm-up protocol consisting of increasing intensity isometric contractions as well as submaximal (ie, 50–70% of maximal effort) concentric contractions at 60°/s and 120°/s to prepare the muscles for maximal effort. Further familiarisation to concentric contractions will be performed before testing trials. For concentric contractions, the amplitude of the movement will be tailored to each individual’s capacities, allowing to manage individuals with limited range of motion due to mechanical constraints. The testing protocol will include three maximal voluntary isometric contractions with additional trials conducted if there is a significant disparity (>5%) between efforts, two series of three slow (60°/s) concentric maximal contractions (the return to initial position being performed by the dynamometer while the participant is fully relaxed) and two other series of three faster (120°/s) concentric maximal contractions. Two-min breaks will be provided between each set, with a 1 min between warm up trials and the testing session.
Measures obtained during strength testing will be presented as peak torque (Nm) and torque normalised to individual weight (Nm/kg). The maximal value obtained in each condition will be retained for presentation and analysis. For testing the quadriceps muscles, participants will be positioned with the knee adaptor of the dynamometer set at a 70° angle (0° being horizontal/full extension of the knee joint) for isometric contractions. The backrest will be set at 90°, the straps for the torso used and a strap will be placed over the thigh of the tested leg. For plantar-flexor strength, an ankle/foot adaptor will be used, with participants lying prone on the backrest and the foot placed in the dynamometer at a 10° angle for isometric contractions (0° being vertical/tibia to foot plane at a right angle). In cases where lying prone leads to discomfort around the knee joint, a soft cloth or pillow will be used under the knee. To evaluate hamstring strength, participants will lie prone with a 6-cm Pilates pad (Pilates pad simple #2650728, Decathlon, Villeneuve, France) placed under the knee to ensure optimal positioning. The backrest will be set at a 100°, and isometric testing will be conducted at a 70° knee angle (0° being horizontal/full extension of the knee joint).
Cost of walking
The metabolical cost of walking will be assessed using a treadmill (ADAL 3D-C, Medical Development, Varces Allieres et Risset, France) equipped with safety bars and the same gas exchange and heart rate monitoring setup as during the CPET and 6-min walking test. In the case of reliance on a cane or discomfort when walking on the treadmill, participants with CP will be allowed to use the bars, preferably those in front, as they minimise the offloading of weight through the arms. Participants will be instructed to choose their preferred walking speed, representing the speed they would typically use when walking outdoors. During the test, participants will walk at three different velocities, 100%, 150% and 120%, of their preferred walking speed. Each speed will be maintained for 3 min or longer if oxygen consumption has not stabilised. A 3-min seated rest will follow every condition. The break period will be prolonged if the oxygen consumption and heart rate do not stabilise.
The cost of walking will be calculated from the respiratory exchange ratio (RER), average speed and average oxygen consumption during steady state walking using the following formula57 :
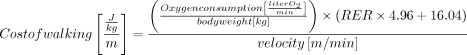
The cost of walking will be expressed normalised to mass and distance (J.kg−1.m−1), and gross oxygen consumption will be normalised to VO2peak (%). This comprehensive assessment will provide valuable information about the metabolic demands of walking at different speeds, allowing for a better understanding of functional limitations and energy expenditure in individuals with cerebral palsy.
Data analysis
In all statistical analyses, significance will be set at p ≤0.05. For our primary objective, data will be treated as a time series and evaluated using Generalized Estimating Equations, a two-way analysis of variance (ANOVA)-like non-parametric test that was also used in the paper by Varesco, et al.28 A time, group and interaction term will be used to investigate the evolution of neuromuscular parameters. All non-time series data (baseline data and all other tests) will either be treated using one-way ANOVA’s or non-parametric tests such as the Kruskal-Wallis test depending on normality of data. Post hoc testing will in either case be Bonferroni corrected. Data analysis will be conducted using R,41 and the code will be available in an online repository.
Ethics and dissemination
The study was approved by the French Ethical board (approval #2022-A02510-43). In accordance with the methods outlined, all participants will undergo evaluation by a medical professional and will be monitored closely throughout the study. An echocardiogram will be performed at rest for healthy participants and at rest and under physical stress during the VO2peak test for participants with CP. Thus, the most intense efforts will be conducted under medical supervision, ensuring participant safety. Medical personnel will be present during all testing sessions, further ensuring the well-being of participants. Participants will receive a summary of their measurements on completion of the study, empowering them with insights into their mobility. Participants have the option to opt out of this part of the protocol if they experience discomfort or spasticity. Alternatively, we can modify the protocol to include only pre- and post-task failure neuromuscular evaluations, although with a reduction in analytical power on our end.
Our dissemination plan includes publishing findings as scientific peer-reviewed articles, presenting them at conferences with relevant clinical professionals and preparing a layman review for all study participants. The number of papers published will depend on word-count limitations and the need for clarity in presenting the multifaceted data. Given the complexity of the data obtained over the 4 days of testing, we believe that a single article may not adequately present and discuss all findings. Therefore, we anticipate multiple papers, with collaborators contributing to each based on the Vancouver protocol (International Committee of Medical Journal Editors). We envision consistent first and last authors across all manuscripts. In order to facilitate a comparison across papers, we will code every participant with either a number or a letter, allowing the reader to follow a participant, not only in the individual paper, but across all papers.
Data management will adhere to European General Data Protection Regulation regulations, with all data stored using participant numbers and encrypted keys on a secure drive. French regulations require a paper trail for consent forms, completed measurements and adverse events, which will be stored in a locked facility. Project progress and data updates will be maintained on the Open Science Framework under the first author’s name. Data will be available on reasonable request. We have adhered to SPIRIT guidelines in the writing of this protocol.
Ethics statements
Patient consent for publication
Acknowledgments
We would like to thank Giorgio Varesco for the artwork in Figure 2.
References
Footnotes
X @gravholta
Contributors BF and TL initiated the study design. AG, BF, DR, AIB, GYM and TL conceived the study. AG, BF, DR, GM and TL conceived the study setup and methods included. AB refined the methods. AG, BF, DR, NZ, HB, LF, LE, AIB, GYM and TL contributed to the refinement of the study protocol and the writing of the protocol paper and approved the final manuscript. TL is the guarantor.
Funding The group led by TL received an internal grant from the university research foundation (Fondation Université Jean Monnet) to cover costs associated with the study and for the PhD scholarship. The funding source had no role in the design of this study and will not have any role during its execution, analysis, interpretation of the data or decision to submit results. The University Hospital of Saint-Étienne has promoted this study.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.




