Article Text
Abstract
Purpose The Management of Post-transplant Infections in Collaborating Hospitals (MATCH) programme, initiated in 2011 and still ongoing, was created to 1) optimise the implementation of existing preventive strategies against viral infections in solid organ transplant (SOT) recipients and allogenic haematopoietic stem-cell transplant (HSCT) recipients and 2) advance research in the field of transplantation by collecting data from a multitude of sources.
Participants All SOT and HSCT recipients at Copenhagen University Hospital, Rigshospitalet, are followed in MATCH. By February 2021, a total of 1192 HSCT recipients and 2039 SOT recipients have been included. Participants are followed life long. An automated electronic data capture system retrieves prospective data from nationwide registries. Data from the years prior to transplantation are also collected.
Findings to date Data entries before and after transplantation include the following: biochemistry: 13 995 222 and 26 127 817; microbiology, cultures: 242 023 and 410 558; other microbiological analyses: 265 007 and 566 402; and pathology: 170 884 and 200 394. There are genomic data on 2431 transplant recipients, whole blood biobank samples from 1003 transplant recipients and faeces biobank samples from 207 HSCT recipients. Clinical data collected in MATCH have contributed to 50 scientific papers published in peer-reviewed journals and have demonstrated success in reducing cytomegalovirus disease in SOT recipients. The programme has established international collaborations with the Swiss Transplant Cohort Study and the lung transplant cohort at Toronto General Hospital.
Future plans Enrolment into MATCH is ongoing with no planned end date for enrolment or follow-up. MATCH will continue to provide high-quality data on transplant recipients and expand and strengthen international collaborations.
- TRANSPLANT MEDICINE
- INFECTIOUS DISEASES
- Bone marrow transplantation
- EPIDEMIOLOGY
- IMMUNOLOGY
- VIROLOGY
Data availability statement
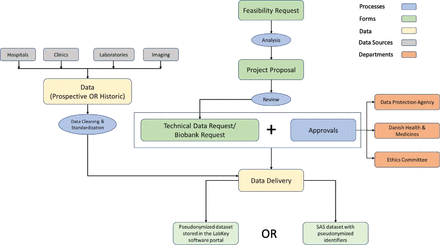
Data are available upon reasonable request. Data are available on reasonable request. MATCH data are open for researchers and data access is regulated by the MATCH Steering Committee. Additionally, the research project must have all required approvals by the Danish regulatory boards according to the type of project. For more details on how to get involved, see figure 3 and the website: (https://www.persimune.dk/How-to-get-involved)
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Management of Post-transplant Infections in Collaborating Hospitals (MATCH) programme is an unselected prospective cohort with complete enrolment, encompassing all transplant recipients at Rigshospitalet, Copenhagen University Hospital, Denmark.
All patients followed in MATCH have a civil registration number, and linkage to the Danish civil registration system (CRS) ensures almost complete life-long follow-up despite patients being transferred to other centres in Denmark.
The Danish CRS allows for linkage to multiple nationwide registries containing data on, for example, biochemistry, pathology, microbiology, imaging, prescriptions and hospital contacts, including ICD-codes for diagnoses and procedures, both from the period before and after transplantations.
MATCH patients, who have given consent to sampling, have provided whole blood samples, plasma samples, bronchoalveolar lavage and faeces samples to an extensive biobank.
MATCH enrols patients from a single centre, reducing the generalisability of findings.
Introduction
The first transplantation worldwide was performed in 1954 and the first in Denmark in 1964.1 2 The field has developed significantly since then with better surgical technique, a better understanding of transplant immunology, the development of immunotherapy, and several other advances in the field of medicine. In spite of these advances, transplantations are still associated with reduced life expectancy, with cancer, allograft rejection, and infectious complications being the most important causes.3
Transplant procedures remain relatively rare, underscoring the importance of systematical gathering of knowledge from expansive groups or cohorts. Many aspects in the field of transplantation, including prevention of infectious disease, lack consensus on best practices due to a paucity of strong evidence.4–6 Therefore, the Management of Post-transplant Infections in Collaborating Hospitals (MATCH) programme was developed at the Copenhagen University Hospital—Rigshospitalet, Denmark.
The prospective MATCH programme was initiated in 2011. It was created for two main reasons: first, to optimise the implementation of existing preventive strategies against viral infections in solid organ transplant (SOT) recipients and haematopoietic stem-cell transplant (HSCT) recipients, and second, to advance research in the field of transplantation by creating an overarching database collecting data from a multitude of data sources. The clinical part of MATCH seeks to improve outcomes of SOT patients and has succeeded in reducing cytomegalovirus (CMV) disease among other things by implementing an electronic clinical support tool ensuring critical follow-up on both screening samples taken and not taken.3 The MATCH cohort has been the basis of more than 50 publications published in peer-reviewed journals so far.
This article aims to describe the profile of the MATCH cohort and provide transparency on its organisation and the data available, thereby nurturing further research and enhancing collaborations.
Cohort description
MATCH organization
MATCH is anchored in the Centre of Excellence in Health, Immunity and Infection (CHIP), at Copenhagen University Hospital—Rigshospitalet, Denmark. MATCH is led by a steering committee with two chairmen, a representative from each transplant department, a representative for the paediatric transplant recipients and two representatives from the CHIP. The steering committee acts as a governing body, overseeing scientific and clinical operations in MATCH.
All transplant recipients from Rigshospitalet are systematically enrolled in MATCH when donors and recipients are matched. This includes all patients receiving a lung and/or liver transplantation in Denmark and all recipients of HSCT, kidney and heart transplantation from Eastern Denmark. Patients have been enrolled prospectively since 2011. Additionally, all transplant recipients from January 2004 to 2010 at the MATCH departments have been added retrospectively to the MATCH programme.
Data infrastructure
The MATCH database is operated by CHIP and embedded into the data structure of the Centre of Excellence for Personalised Medicine of Infectious Complications in Immune Deficiency (PERSIMUNE). CHIP is responsible for overall operation, data processing, stability and access control of the MATCH database.
The PERSIMUNE Datawarehouse (DWH) receives data on patients in the MATCH cohort from a multitude of sources (figure 1). This is enabled by the Danish civil registration system (CRS).7 Every Danish resident is registered in the CRS with a unique 10-digit Civil Personal Register (CPR) number. The CPR number is used in all Danish registers and thereby allows linkage of data across multiple sources. Denmark has multiple national health registers, linked by the CPR number. Provided that the relevant approvals have been obtained from the research legal department data specific for individual research projects can be imported into the DWH and thereafter linked with other data already available in the DWH in a pseudonymised combined data extract. An example is the import of data on consecutive pulmonary lung function tests on lung transplant recipients for a specific lung transplant project.
Overview of MATCH data sources and flow. Data are collected from various local and national sources and incorporated into the PERSIMUNE Datawarehouse from where data on MATCH patients can be requested. CLASS, Classification of death causes after transplantation; MATCH, Management of Post-transplant Infections in Collaborating Hospitals; PERSIMUNE, Centre of Excellence for Personalised Medicine of Infectious Complications in Immune Deficiency; MADS, Mikrobiologisk Afdelings Data System; LABKA, The Clinical Laboratory Information System; EPM, Electronic Patient Medicin .
Data sources
DWH receives data from many of the national health registers, including the National Patient Register, the National Organ Donor Database, the Danish Hospital Medication Register, the Danish Microbiology Database (MiBa), the Danish Pathology Register and the Cause of Death Register. More specifically, DWH receives data on investigations performed as a part of clinical practice from the following: LABKA I (2005–2009) and LABKA II (2009–) provides data on biochemistry, and MEDCOM (2004–) provides data on microbiology, pathology and additional biochemistry. Data on medication and all outpatient prescriptions are provided by EPM1 (2005–2020) and EPM3 (2012–2016), and Sundheds-databanken provides data on hospital contacts, procedures and diagnosis codes. Data on demographics, deaths and emigration are obtained from the CRS and the Cause of Death Register (2010–). MADS (2005–), a local database, provides additional microbiology data. The Radiology Information System / Picture Archive and Communication System (RIS/PACS) (2005–) is a local data source that provides data on imaging. The DWH obtains clinical data from electronic medical files and genetic data from other internal sources.
Quality assurance
Before being incorporated into the data stream, data from each data source are checked by a five-step procedure, involving source identification, obtaining documentation, clarification of which data columns/types to collect, establishment of data harvest and finally, an assessment of the data harvest. An ongoing data cleaning and quality assurance (QA) process are performed. This process includes, but is not limited to, generating QA tables, generating histograms for analysis, triangulation/cross-validation of data, defining rules for clean-up, testing and validating clean-up rules, defining rules for monitoring data and implementing monitoring rules and surveillance. Furthermore, clinical biochemistry and microbiology data are grouped according to sample material and type of analysis. An example of how a biochemistry variable undergoes QA is available in the online supplemental material Example of Data Cleaning.
Supplemental material
Data enrichment
After importing and cleaning of data in the DWH, PERSIMUNE performs additional data enrichment, combining data variables from data sources to create calculated variables based on standardised definitions. An example is the calculation of a Charlson Comorbidity Index (CCI).8 Another example is a CMV infection algorithm used to define if a transplant recipient has a CMV infection. The algorithm checks if a recipient has two consecutive plasma CMV PCR taken within 14 days of each other with a viral load ≥273 IU/mL or one sample with a viral load ≥2730 IU/mL.
Clinical data
In overall numbers until 28 February 2021, the following data are available from the MATCH cohort: 464 783 medical diagnosis codes, 314 961 data entries on medication before recipient transplantation and 367 839 after transplantation. Biochemistry data are available with 13 995 222 entries before transplantation and 26 127 817 after. Microbiology data are available with 242 023 culture results before transplantation and 410 558 after, and data on other microbiology analyses performed are available with 265 007 results before transplantation and 566 402 after. Data on pathological examinations are available on 1 70 884 samples before and 200 394 after transplantation.
Some research projects result in additional data being incorporated into the DWH and are available for other research projects on approval. One such example is the Classification of death causes after transplantation (CLASS) project.9 CLASS is a methodology used to systematically and reliably determine and classify an accurate cause of death in all transplant recipients, than otherwise obtainable from Danish causes of death register.
Genetic data
In 2017, after ethical approval, samples from all patients in the MATCH cohort, with available material for analysis at that time point, were genotyped using the Infinium Global Screening Array-24 v1.0 from DeCode. In 2019, all patients in the MATCH cohort with available material for analysis were genotyped at 770 558 single-nucleotide polymorphism loci using a custom array from Affymetrix, designed to enrich genes relating to immune dysfunction. In total, 2431 (75.2%) transplant recipients have been genotyped.
Biobank
In 2015, a biobank for future research was established by PERSIMUNE, in collaboration with Rigshospitalet and the Department of Clinical Immunology, with samples being continuously collected from patients in the MATCH cohort (among others) who gave their consent. A total of 1731 patients have provided samples to the biobank. Blood samples are collected before transplantation and 1 year after transplantation, with 1207 (69.7%) recipients having contributed at least one whole blood sample. In 2016, faeces samples were added to the collection scheme for two transplant recipient groups: HSCT and kidney transplant recipients with living donors. For HSCT recipients who have consented to this, faeces samples are collected pretransplantation and at days 7, 14, 21, 28 and 180 after transplantation. For kidney transplant recipients, faeces samples are collected pretransplantation, within 3 months and 3 months after transplantation. Since 2021, bronchoalveolar lavage fluid is collected and stored on every bronchoscopy performed in lung transplant recipients who have consented to this.
Cohort participants
From 2011 to 28 February 2021, 3231 transplant recipients have been prospectively enrolled in the MATCH programme with the majority being HSCT or kidney transplant recipients (figure 2A). The number of patients enrolled has been stable over time, with a slight upwards trend (figure 2B)
(A) Number of transplant recipients each year per type of transplantation. (B) Overview of the distribution of recipients by organ type, included in MATCH from January 2011 to February 2021. HSCT, haematopoietic stem-cell transplantation recipients.
Among the 3231 transplant recipients, 4.3% had a re-transplantation. Overall, 59.6% of transplant recipients were male. Age at transplantation was similar across the transplant groups with a mean age of 50 (IQR 35, 60). The proportion of transplant recipients under the age of 18 years was 19.8, 12.5, 10.6, 4.3 and 0.6% for HSCT, liver, heart, kidney and lung recipients, respectively. The donor/recipient CMV and Epstein-Barr Virus (EBV) serostatus in the cohort at baseline are summarised in table 1. There was a CCI score available for 2961 (91.6%) of the transplant recipients with a median score of 1 (IQR of 1–3) at the time of transplantation.
Baseline characteristics of transplant recipients enrolled in MATCH between 2011 and February 2021
Until 28 February 2021, a total of 796/3231 (24.6%) have died during follow-up. In the MATCH cohort, the causes of death in the SOT recipients were cancer (19.1%), graft rejection (18.4%), infections (17.4%), other organ-specific or non-specific causes (15.4%), graft failure (11.7%) and cardiovascular disease (10.0%). For 8% of SOT recipients, the cause of death was unknown.3 For HSCT recipients, death from relapse was the most frequent cause of death (46.0%), followed by graft versus host disease (22.1%), other causes (13.5%) and infections (12.1%). The cause of death was unknown for 6.2% of HSCT recipients.10
The total follow-up time for HSCT and SOT recipients is 5192 and 8840 years, median 2.9 (IQR 0.9–6.2) and 4.3 (IQR 1.8–7.2), respectively. Follow-up of those who are alive is ongoing and independent of graft loss.
Findings to date
With the right regulatory approvals, researchers can get access to the clinical data collected in the MATCH programme. Since 2011 until 2023, clinical data from the MATCH programme have been the basis of more than 50 scientific publications. A full publication list is available in the online supplemental material Full Publication List.
Supplemental material
Cytomegalovirus
The implementation of MATCH succeeded in reducing CMV disease among non-lung SOT recipients as demonstrated by Ekenberg et al, with an adjusted HR of 0.27 [0.11–0.63], p = 0.003, early after implementation, and an adjusted HR of 0.17 [0.06–0.52], p = 0.002, late after implementation, both compared with prior to MATCH.11 Other elements of CMV management in MATCH have also been studied, including the development of antiviral resistance.11–21
Other infections
The epidemiology of a range of other infectious diseases in transplant recipients has also been studied based on the MATCH cohort with findings among others: a high incidence of invasive aspergillosis the first 3 months after CMV infection and a high incidence of herpes vira (CMV, EBV, herpes simplex type 1 and 2, and varicella zoster) infections.22–30
Classification of death causes after transplantation
The CLASS study aimed to develop a method to improve our understanding of the cause of death in transplant recipients, thereby helping identify emerging trends and health challenges in transplant recipients. The method uses trained investigators to complete a case report form on fatal cases, which is then assessed by two external reviewers, and if not in agreement are further evaluated by an expert panel.9 This method was used in another study, finding a trend towards lower incidence of death from cardiovascular disease, graft failure and cancer over time, while non-organ-specific causes did not decrease.3 The method also identified a sub-group of transplant recipients with an increased risk of death to cancer or cardiovascular disease, namely, patients with either pre- or postdiabetes mellitus.31
Vitamins
Some studies also examined the role of vitamins A, E and D in the acute graft versus host response in HSCT patients.32–34
Microbiome
The role of the gut microbiome in transplant recipients has also been investigated.35–38 One landmark study showed that the composition of the pretransplant gut microbiome is associated with the risk of acute graft versus host disease in HSCT patients.39
Cancer and PTLD
Cancer in transplant recipients has been a focus area with one study finding an increased risk of de novo or secondary cancers after solid organ or allogenic haematopoietic stem cell transplantation compared with the general population.40 One study examined the predictive value of EBV DNA in detection of posttransplant lymphoproliferative disorders (PTLD) in transplant recipients and investigated how the addition of other variables in the model could improve the prediction of PTLD.41 Another study examined early- and late-onset PTLD among adult kidney and liver transplant recipients,42 and in the same year, a risk score for PTLD in SOT recipients was developed and validated.43
Other research areas
Other studies based on the clinical data from the MATCH programme investigated the clinical utility of different medical devices and scoring systems, different biomarkers such as ST2 and CRP, treatment options, and the role of immune reconstitution and function in transplant recipients.43–54
Partnerships and collaborations
MATCH has several international research collaborations. MATCH is collaborating with a transplant cohort based at the Toronto General Hospital regarding infectious complications in lung transplant recipients. MATCH is also collaborating with the Swiss Transplant Cohort Study aiming to merge more than 10 000 SOT recipients from both cohorts to evaluate and compare outcomes of different strategies against CMV infection.55 Representatives from MATCH have also worked with the CMV Resistance Working group, a subgroup of the CMV Drug Development Forum, on definitions of resistant and refractory CMV in transplant recipients.19
Collaboration
MATCH encourages both local and international collaborations. Research projects seeking to use data from MATCH must be approved by the MATCH Steering Committee. For more details, please see data sharing statement.
Future perspectives
The MATCH programme has existed for 13 years, contributing a great amount of data in high granularity and of high quality that can be used for research purposes. These data have been used in a series of scientific publications. Future endeavours involve expanding and strengthening international collaborations to improve the quality, generalisability and utility of evidence in the transplantation field. Large collaborations are essential to overcome limitations posed by the rarity of transplantations.
Further Details
Strengths and limitations of this study
MATCH is an unselected prospective cohort with complete enrolment, encompassing all transplant recipients at Rigshospitalet, Copenhagen University Hospital, Denmark.
All patients followed in MATCH have a civil registration number, and linkage to the Danish civil registration system ensures almost complete life-long follow-up despite patients being transferred to other centres in Denmark.
The Danish civil registration system allows for linkage to multiple nationwide registries containing data on e.g. biochemistry, pathology, microbiology, imaging, prescriptions and hospital contacts, including ICD-codes for diagnoses and procedures, both from the period before and after transplantation.
MATCH patients, who have given consent to sampling, have provided whole blood samples, plasma samples, bronchoalveolar lavage and faeces samples to an extensive biobank.
MATCH enrols patients from a single centre, reducing the generalizability of findings.
How to obtain data for research in the Management of Post-transplant Infections in Collaborating Hospitals. An optional feasibility request can be made to evaluate if data of interest is available. When the researcher has confirmed that the data of interest is available, the next step is to submit a project proposal. Once the project has been approved, a data request must be made defining the patient group, all data elements required, as well as all relevant regulatory approvals. Data will be delivered in a pseudonymised form. Finally, all collaborators are asked to contribute to the ongoing data cleaning, standardisation and enrichment of data used in their research project.
Data availability statement
Data are available upon reasonable request. Data are available on reasonable request. MATCH data are open for researchers and data access is regulated by the MATCH Steering Committee. Additionally, the research project must have all required approvals by the Danish regulatory boards according to the type of project. For more details on how to get involved, see figure 3 and the website: (https://www.persimune.dk/How-to-get-involved)
Ethics statements
Patient consent for publication
Ethics approval
This project was approved by the Centre for Regional Development (Journal-nr.: R-21018786), which approves projects seeking to retrospectively access treatment systems or electronic health records for research purposes on behalf of the Danish Data Protection Agency.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @Sadaf Zahid
Contributors Guarantor: MH; conceptualisation and writing: FVLE, CGC and MH; formal analysis: FVLE, SZ and NN; review, edit and revision: KSM, NN, DDM, CM, FG, HS, MP, NAS, SSS, JM, VBC and JL. All authors have read and agreed to the published version of the manuscript.
Funding Research activities in the Management of Post-transplant Infections in Collaborating Hospitals (MATCH) program are funded by the Danish National Research Foundation (Grant number DNRF 126). Copenhagen University Hospital—Rigshospitalet, Denmark, supports the MATCH program and its quality assurance and patient treatment.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.