Article Text
Abstract
Objectives Leukaemias and lymphomas are among the most prevalent and significant cancers in Australasia and Oceania. This study aims to examine the burden of leukaemias/lymphomas and its temporal trend in Australasia and Oceania from 2010 to 2019.
Design Epidemiological study
Methods Data from the Global Burden of Disease (GBD) 2019 were used to examine the burden of leukaemia/lymphoma key subtypes (acute lymphocytic leukaemia (ALL), acute myeloid leukaemia (AML), chronic lymphocytic leukaemia (CLL), chronic myeloid leukaemia (CML), Hodgkin-lymphoma (HL) and non-Hodgkin’s lymphoma (NHL)) by sex and 5 year age groups (from <5 years to 85 years+), in terms of incidence, prevalence, disability-adjusted life years (DALYs) and deaths. Estimated average percentage changes were calculated to assess the temporal trends of leukaemia/lymphoma burden (incidence/prevalence/DALYs/deaths) from 2010 to 2019 in Australasia and Oceania.
Results AML and NHL were the leading causes of leukaemia/lymphoma burden in both regions. Age-standardised rates (ASRs) for AML versus NHL in Australasia were: incidence 4.72 versus 19.06, DALYs 89.01 versus 161.68 and deaths 4.15 versus 8.02 per 100 000 population. ASRs for AML versus NHL in Oceania were: incidence 1.36 versus 1.08, DALYs 49.16 versus 38.30 and deaths 0.91 versus 0.98 per 100 000 population. From 2010 to 2019, Australasia observed an increasing trend in incidence/prevalence/deaths across most leukaemias/lymphomas and increasing/stable trend in DALYs for AML/CLL/NHL, while Oceania observed increasing trends in incidence/prevalence/DALYs for CLL/NHL and stable trends in all outcomes (except for prevalence (stable)) for AML. Contrasting mortality trends for ALL/CML/HL were observed between the two regions (increasing/stable in Australasia and decreasing in Oceania). Statistically significant differences were observed in disease burden trends between sexes, with males experiencing a greater increase (or smaller decrease) in the burden for AML in both regions.
Conclusions Different temporal trends in leukaemia/lymphoma burden observed in two closely situated geographic regions with different sociodemographic indices highlight the necessity for region-specific intervention strategies to enhance the access to innovative disease treatments, reducing leukaemia/lymphoma burden.
- Lymphoma
- Leukaemia
- EPIDEMIOLOGIC STUDIES
Data availability statement
All data relevant to the study are included in the article and uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
We compared the disease burden and temporal trends in two geographically similar regions with differing sociodemographic indices and performed a sensitivity analysis using the world standard population, which facilitated the comparison of trends between Australasia and Oceania, and to other regions.
Using region-specific data helps reflect disease burden distributions and trends across sexes and age groups more comprehensively and accurately.
A direct comparison between Australasia and Oceania is limited due to considerable differences in population distributions and risk factors between the two regions.
Analysing aggregate data made it challenging to discern the impact of various exposures to disease outcomes, thus trends should be interpreted with caution.
Introduction
Haematological malignancies, including leukaemias and lymphomas, arise from the uncontrolled proliferation of cells in the lymphatic or circulatory systems. Based on the Global Burden of Disease, Injuries and Risk Factors Study (GBD) 2019, which provides the most comprehensive estimates of global disease and injury burden to date, haematological malignancies contribute to a considerable proportion of the global disease burden attributed to cancer.1–3 Globally, leukaemias and lymphomas contributed to 11.7 million and 8.2 million disability-adjusted life years (DALYs) in 2019, respectively.2 Studies exploring the temporal trend in haematological malignancies across countries using data from the GBD 2019 study have found that over a 30 year period, age-standardised mortality/DALYs have declined, against a background of increasing incident/prevalent burden. However, the distribution of disease burden and temporal trends in leukaemias/lymphomas varies across geographic regions and varying levels of socioeconomic development.1 2 4 5 Differences in disease burden across regions of high/low socioeconomic development were largely attributed to social and environmental factors including poverty, educational attainment and access to healthcare.1 2 4 These large disparities in the healthcare system highlight the need for population-based epidemiological studies in both high and low-income and middle-income countries to inform public health policy and healthcare delivery planning.1 2 4 5 Importantly, no studies have systematically explored trends in disease incidence/prevalence or burden of leukaemias/lymphomas for Australasia and Oceania.1 2 Epidemiological studies comparing these two Pacific regions are particularly beneficial given the considerable socioeconomic, cultural and ethnic differences between these regions.3 As such, a comparison of contemporaneous leukaemia/lymphoma trends between Australasia and Oceania may facilitate the understanding of healthcare disparities, the impacts of sociodemographic factors on disease occurrence and outcomes and the role of healthcare infrastructure in managing these cancers. Moreover, although data on leukaemia and lymphoma burden are often reported in regional cancer registry reports, and also publicly available in the GBD data set, research specifically focusing on trends in haematological malignancies in Australasia and Oceania are scarce.3 6–8 This gap in the literature underscores the importance of region-specific research to better understand these trends and inform policies tailored to these regions. Ultimately, such a study would inform future research, public healthcare planning strategies and policies aimed at reducing the burden related to leukaemia/lymphoma in Australasia and Oceania—the two regions populated with Indigenous people, closely geographically located but varied sociodemographic factors.6–9
Hence, this study aims to (1) examine the prevalence, incidence, mortality and DALYs attributed to leukaemias and lymphomas by sex and age groups and (2) explore the temporal trend in these metrics for leukaemias and lymphomas from 2010 to 2019 in Australasia and Oceania regions using GBD 2019 data.
Methods
Data source
We extracted data from the GBD 2019 and performed a secondary analysis in the current study.9 All data were collected using Global Health Data Exchange (GHDx) query tool (http://ghdx.healthdata.org/gbd-results-tool).3 10 Details pertaining to the collection, processing and generation of the GBD 2019 study data set have been described elsewhere.2 3
Case definition
The definition of leukaemias and lymphomas used in the GBD 2019 study has been defined previously using International Classification of Diseases (ICD) codes (online supplemental appendix A).2 3 Leukaemia is typically classified by the type of white blood affected (lymphocytic or myeloid) and disease progression (acute or chronic).11 Key leukaemia subtypes include acute lymphocytic leukaemia (ALL), acute myeloid leukaemia (AML), chronic lymphocytic leukaemia (CLL) and chronic myeloid leukaemia (CML).11 Lymphomas are categorised based on the type of lymphocyte affected, with key lymphoma subtypes including non-Hodgkin’s lymphomas (NHL) and Hodgkin’s lymphoma (HL).12
Supplemental material
Population and outcome
In this study, we assessed the burden of disease (including prevalence, incidence, deaths and DALYs) of leukaemias and lymphomas by subtype in Australasia and Oceania from 2010 to 2019. In line with GBD 2019 definitions, Australasia was defined as Australia and New Zealand, and Oceania (18 countries) was defined as American Samoa, Cook Islands, Fiji, Guam, Kiribati, Marshall Islands, Micronesia, Nauru, Niue, Northern Mariana Islands, Palau, Papua New Guinea, Samoa, Solomon Islands, Tokelau, Tonga, Tuvalu and Vanuatu.3
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Statistical analysis
A descriptive analysis was performed to characterise the regional burden of leukaemias and lymphomas (by subtype). The number of prevalent and incident cases, deaths and DALYs were used to derive age-standardised rates (ASRs) (per 100 000 population) in Australasia and Oceania from 2010 to 2019. ASRs were calculated to exclude the impact of age structure on overall population prevalence, incidence, mortality and DALYs as the number of cases, deaths and DALYs of cancers varies greatly across different age groups.1 10 The ASR per 100 000 population is the weighted-average of age-group-specific rates, that is,
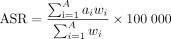 (1)
(1)
where  is the age-group specific rate i and
is the age-group specific rate i and 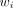 is the weight of age group i of the population.10 For the purposes of the base-case analysis, the number of outcomes (incidence, prevalence, deaths or DALYs) occurring for each 5 year age group (<5 years to 85+), were divided by the number of individuals estimated in each age group for Australasia (or Oceania) to estimate age-group-specific rates for each year (
is the weight of age group i of the population.10 For the purposes of the base-case analysis, the number of outcomes (incidence, prevalence, deaths or DALYs) occurring for each 5 year age group (<5 years to 85+), were divided by the number of individuals estimated in each age group for Australasia (or Oceania) to estimate age-group-specific rates for each year ( ). This was multiplied by the proportional distribution (
). This was multiplied by the proportional distribution (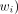 ) of individuals in each age group to estimate the ASR.
) of individuals in each age group to estimate the ASR.
The estimated average percentage change (EAPC) in ASRs was estimated to assess temporal trends in prevalence, incidence, mortality and DALYs of leukaemias and lymphomas (by subtype) over a 10 year period (2010–2019, inclusive). EAPCs were calculated through generalised linear regression modelling with a Gaussian family and log-link to estimate trends: y =
α
+
β
*x +
ε
, where y is ln(ASR) and x is the calendar year. The EAPC was expressed as 100*( −1).1 If the estimated β-coefficient and its 95% CI were both positive, it indicated an upward trend in ASR, whereas a negative β-coefficient and its 95% CI indicated a downward trend in ASR.1 10 Otherwise, the temporal trend in outcomes was assumed to be stable. P-values<0.05 were considered statistically significant.
−1).1 If the estimated β-coefficient and its 95% CI were both positive, it indicated an upward trend in ASR, whereas a negative β-coefficient and its 95% CI indicated a downward trend in ASR.1 10 Otherwise, the temporal trend in outcomes was assumed to be stable. P-values<0.05 were considered statistically significant.
All calculations were performed using Stata V.17.0 statistical software (Stata Corp, College Station, TX).
Sensitivity analysis
As described above, ASRs were calculated in our base-case analysis based on the estimated population size across 5 year age groups for Australasia and Oceania. To allow for comparability between trends in Australasia and Oceania, a sensitivity analysis was performed by applying the world standard population weights to age-group-specific rates for key outcomes estimated in our base-case analysis.13
Results
The leukaemia and lymphoma burden in terms of incidence, prevalence, DALYs and deaths across Australasia and Oceania and associated temporal trends are summarised in tables 1 and 2, respectively. Tables 3 and 4 summarise trends in leukaemia and lymphoma burden by sex. Figure 1 summarises the leukaemia and lymphoma burden in terms of incidence, prevalence, DALYs and deaths across Australasia and Oceania, by age group, and sex in 2019, respectively. Figure 2 illustrates the trends in leukaemia and lymphoma burden by subtypes during the period 2010–2019.
Age-specific rates at regional level by sex in Australasia and Oceania in 2010 and 2019. This figure represents age-specific rates (per 100 000 population) of incidence, prevalence, disability-adjsuted life years (DALYs) and deaths in Australasia (a, b, c, d, e, f, g, h), and in Oceania (i, j, k, l, m, n, o, p) by sex in 2010 and 2019, repsectively. ALL, acute lymphocytic leukaemia; AML, acute myeloid leukaemia; CLL, chronic lymphocytic leukaemia; CML, chronic myeloid leukaemia; DALY, disability-adjusted life year; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma.
Trends of disease burden based on age-standardised rates in Australasia and Oceania from 2010 to 2019. ALL, acute lymphocytic leukaemia; AML, acute myeloid leukaemia; CLL, chronic lymphocytic leukaemia; CML, chronic myeloid leukaemia; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma.
Results of ASRs and EAPCs for key outcomes across leukaemia and lymphoma subtypes in Australasia in 2010 and 2019
Results of ASRs and EAPCs for key outcomes across leukaemia and lymphoma subtypes in Oceania in 2010 and 2019
Trends for key outcomes across leukaemia and lymphoma subtypes in Australasia in 2010 and 2019 by sex
Trends for key outcomes across leukaemia and lymphoma subtypes in Oceania in 2010 and 2019 by sex
Overview of disease burden
Leukaemia and lymphoma burden in Australasia
Across Australasia, the greatest contributor to incidence of leukaemia and lymphoma in 2019 was CLL (ASR: 5.22 per 100 000) and NHL (ASR: 19.06 per 100 000), respectively. ALL accounted for the largest share in paediatric leukaemia incidence, whereas CLL accounted for the largest share of incident cases for adult leukaemias. NHL (ASRs: 161.68 DALYs and 8.02 deaths per 100 000), AML (ASRs: 89.01 DALYs and 4.15 deaths per 100 000), followed by CLL, contributed to the greatest proportion in terms of DALYs and mortality burden in 2019. NHL was the most prevalent haematological cancer in 2019. Following stratification of disease burden by 5 year age band and sex, the incidence, prevalence, DALY and mortality rates across 5 year age groups generally increased with ascending age across leukaemia/lymphoma subtypes for Australasia (see figure 1 and online supplemental appendix B). Age-specific incidence/prevalence and DALY rates for ALL and HL followed a bimodal pattern.
Leukaemia and lymphoma burden in Oceania
In Oceania, AML and ALL drove leukaemia burden (incident/prevalent/DALYs/deaths) and NHL was the key contributor to the incident lymphoma burden (ASR: 1.08 per 100 000) in 2019. HL (ASR: 0.81 per 100 000) drove prevalent lymphoma burden. Regarding the burden of disease (DALYs/deaths), AML (ASRs: 49.16 DALYs and 0.91 deaths per 100 000) and NHL (ASRs: 38.30 DALYs and 0.98 deaths per 100 000) were the largest contributors in 2019. Disease burden across age-groups and sexes for Oceania were broadly comparable with Australasia. That is, incident/prevalent burden, as well as DALYs/deaths, increased with increasing age and for male sex (Table 1).
Temporal trends in leukaemia and lymphomas (base-case analysis)
Australasia
The incidence and prevalence of haematological malignancies across Australasia increased significantly over a 10 year period (2010 to 2019, inclusive) (p<0.001). Temporal increasing trends in incident/prevalent cases were largely driven by CLL, ALL and CML (EAPCs ranging from 2.16% to 2.87%) (all p<0.001). Notably, the EAPC and associated 95% CI for leukaemia and lymphoma incidence was broadly comparable between sexes with the exception of CML and HL (greater for females), and AML (greater for males). Regarding prevalent burden, EAPCs in males were found to be higher for AML, and lower for HL relative to females.
DALY burden (per 100 000) increased significantly for CLL (EAPC: 1.74%) and AML (EAPC: 1.15%) over time, whereas significant reductions in DALY burden attributed to CML (EAPC: −1.23%), HL (EAPC: −0.40%) and ALL (EAPC: −0.36%) were observed (all p<0.05). DALY burden attributed to NHL remained stable over time (both sexes). Male sex drove temporal reductions in DALYs (ALL, CML and HL), whereas the only significant reduction in DALY burden for females was for HL. Although AML and CLL DALY burden increased for both sexes, the EAPC estimated for AML was of greater magnitude for males relative to females (EAPCs: 1.51% vs 0.69%). Finally, mortality from leukaemias/lymphomas significantly increased over time (EAPCs ranging from 0.75% for ALL to 2.11% for CLL), with the exception of CML and HL (stable). Overall, trends in mortality were comparable between sexes, with the exception of greater increases in mortality over time for AML and NHL for males compared with females (EAPCs: 2.55% vs 1.46%) (table 2).
Oceania
The increased incidence in CLL (EAPC: 1.38%) and NHL (EAPC: 0.92%) was balanced with significantly decreases in ALL (EAPC: −0.45%) and CML (EAPC: −0.58%) incidence. The overall stable trend in HL incidence over time was a result of opposing temporal trends in males (decreasing with EAPC: −0.19%) and females (increasing with EAPC: 0.31%). The overall incidence of AML was stable, driven by females (stable), despite increasing over time for males (EAPC: 0.17%). Although CLL incidence increased over time for both sexes, the EAPC was higher for females. Significant increases in the prevalence of NHL (EAPC: 3.24%), CLL (EAPC: 1.99%) and HL (EAPC: 0.68%) were balanced with reductions in the prevalence of CML (EAPC: −0.76%), ALL (EAPC: −0.56%) and AML (EAPC: −0.21%). Although temporal trends for ALL, CML CLL, HL and NHL prevalence were in the same direction across both sexes (decreasing for ALL/CML and increasing for CLL/HL/NHL), the decline in AML prevalence was driven by females (stable for males). Ultimately, the overall incidence and prevalence of leukaemias/lymphomas across Oceania had remained stable over time.
In terms of disease burden, modest but statistically significant annual reductions in DALYs were observed for ALL, CML and HL (range in EAPCs: −0.55% for HL to −0.88% for CML) across both sexes, whereas the DALY burden attributed to CLL (EAPC: 0.92%) and NHL increased over time (EAPC: 0.26%) (all p<0.001). AML DALY burden over time was stable, and largely driven by males (stable in males while decreasing in females). Finally, with the exception of AML, CLL and NHL, the mortality burden attributed to leukaemias/lymphomas declined over time (range in EAPCs: −0.48% for CML to −0.35% for HL). Between 2010 and 2019, significant declines in mortality burden attributed to ALL and CML, and significantly increased CLL mortality, were estimated across both sexes. However, AML mortality burden increased significantly for males, and remained stable over time for females. Furthermore, the increase in CLL mortality over time was greater for females relative to males. Regarding lymphoma subtypes and sex, the overall decline in HL mortality was driven by males (stable for females), while NHL mortality increased over time for both sexes (Table 2).
Sensitivity analysis
Results of the sensitivity analysis are presented in online supplemental appendix C. While broadly comparable with base-case estimates, discrepancies in the direction of the trend (decreasing, stable or increasing) over time were identified for 20 (42%) out of 48 estimable trends following application of the world standard population.
Discussion
Our study is the first to report trends across a variety of outcomes between Australasia and Oceania over a 10 year period (2010–2019, inclusive) in which considerable changes in clinical practice occurred for cancer.1–3 Discrepancies in disease burden (as indicated by ASRs) across regions, and across age groups and sexes were observed. This is likely attributed to differences in age distribution,1 14 differences in healthcare and cancer surveillance/registration systems, as well as differences in accessing the social determinants of health between high sociodemographic indices (SDI) and low-SDI countries.1 2 For example, recent advances in the treatment of NHL and AML have contributed to improved patient survival, including the development of immune-based cellular and antibody therapies, and small molecule inhibitors.15 16 However, despite improved management of patients with leukaemias/lymphomas in Australasia, the considerable disease burden for both NHL and AML warrants further research to address unmet need. Moreover, NHL contributed the most to mortality burden in Oceania, which may highlight issues in the effective management of NHL in the region. On stratification to explore the distribution of age (5 year age group) and sex (male/female) burden, the observed leukaemia/lymphoma burden was greater for older (vs younger) persons and males (vs females) across both regions. This is in line with other studies exploring patient outcomes in AML, and has been attributed to differences in biological risk factors, lifestyle or environmental factors, and differences in disease treatment and management.17 18 Factors including issues with health systems reporting, differential access to health services between females and males, the interaction between sex and age distribution and gender inequality have also been implicated in contributing to discrepancies in disease burden between sexes across low-SDI and high-SDI countries.1 5 10 However, further studies are recommended to establish and address sex-based drivers of leukaemia/lymphoma burden in countries with low-SDIs or middle-SDIs.1 5 10 19 The greater burden observed in adults and older age groups also highlights the need for age-period-cohort effect analysis to explore age-specific risk factors and examine the effects of age, time period and birth cohort on leukaemia/lymphoma incidence and mortality in these regions.20
The interaction between factors for differences in leukaemia/lymphoma burden trends between Australasia and Oceanian is similarly complex. First, there are considerable differences in ethnic and genetic distribution across regions. For example, CLL is relatively rare among Pacific Islanders (who comprise the majority of Oceanian populations) compared with Caucasian or European peoples (Australasia).21 Second, the majority of countries in Oceania have limited healthcare infrastructure and access to healthcare specialists, as well as limited access to early detection and advanced medical treatments relative to Australasia.6 22 Moreover, although there are several cancer registries in Oceania, the quality and completeness of registration varies.23 For example, cancer registries in Fiji have reported cases under-registration, gender miscoding and variations in coding causes of deaths. Issues regarding the completeness of data and validity of diagnoses have also been reported in Tonga, Cook Islands and Niue. In the US-affiliated islands such as American Samoa and Guam, there is the potential for missing cases due to historical barriers and the lack of resources for diagnosis and staging.6 In contrast, Australasia has well-developed healthcare systems with advanced medical technologies, leading to earlier detection, more effective treatment options and improved survival rates.22 Notably, significant reductions in the CML/HL/ALL DALY burden were observed, and NHL DALY burden remained stable over time which reflect improved outcomes due to changes in patient management and treatment across Australasia.24–27 However, overall, our findings highlight the need to improve prevention strategies to mitigate exposure to lifestyle factors associated with leukaemia/lymphoma, and the efficient allocation of resources among vulnerable population groups for the treatment for leukaemias/lymphomas.28
First, while trends were comparable across both sexes; a greater increase in AML mortality for males was estimated relative to females. As with disparate disease burden between sexes, disparate trends between sexes is consistent with existing studies exploring the leukaemia/lymphoma burden for high-SDI countries.1 10 That is, differences be attributed to differences in tobacco use, occupational exposure to carcinogens and high body mass index.29 Second, the discrepancy between reductions/stable DALY burden (ALL and NHL) against increasing mortality/incidence/prevalence over time may highlight disparate outcomes across age groups.8 24 25 30 ALL follows a bimodal age distribution, with incident/prevalent cases peaking during early childhood and later adulthood (≥50 years), while NHL cases and the risk of mortality (ALL and NHL) increase with age.17 31 32 Therefore, it is likely that while improved disease management and treatment over time have reduced disease burden (DALYs) for younger patients, these changes may not have had an equal impact on reducing mortality burden among older patients.23 This is supported by recent studies exploring ALL and NHL outcomes across Australasia, which found improvements in the management or treatment of disease coincided with greater gains in survival outcomes for younger relative to older patients.8 24 25 30 33 To effectively address the ongoing burden of leukaemias/lymphomas, further research should prioritise exploring trends in disease burden and treatment outcomes across different age groups, with a focus on identifying and addressing the factors contributing to the observed disparities among these groups. Although causes of most leukaemias/lymphomas are unknown, general cancer prevention strategies can target lifestyle factors, such as avoiding tobacco, having a healthy diet and reducing exposure to hazards such as radiation and toxic chemicals to reduce the risk of leukaemias/lymphomas.1 Finally, discrepancies in temporal trends were identified in sensitivity analyses using the world standard population. Notably, disease burden attributed to AML and NHL reduced across both regions in comparison with base-case estimates. This is likely attributed to the variation in population structure in each region, which differs from the standard population.3 13 As sex and age are associated with cancer outcomes, it is possible that EAPCs estimated using the standard population would inadequately capture the impact of region-specific differences in age/sex, and ultimately, overestimate patient survival trends.
A key strength of our study lies in using data from the GBD 2019 study, which to date, provides the latest regional epidemiological distributions and trends of leukaemias and lymphomas.3 Besides, the comprehensive analysis of trends across a variety of key outcomes facilitates an understanding of the impacts of changes in patient management/treatment on patient outcomes and potential areas of unmet need. Furthermore, disease burden and temporal trends in our study were estimated based on region-specific population, which may better reflect disease burden distribution and trend across age groups and sexes more comprehensively and accurately, particularly when regional population structure is incomparable to the world standard population. Finally, restricting the analysis to two geographically similar regions with differing SDI, and performing a sensitivity analysis using the world standard population facilitates the comparison of trends between the two regions (base-case), and to other regions or nations at the global level (sensitivity analysis).
However, several limitations to our analyses warrant mention. First, this study used data from GBD 2019, which does not include data in 2020 and 2021 (which will be available in the latest GBD update). As a result, it may not capture the most recent trends in disease burden up to 2021. Other limitations associated with GBD 2019 data have been described previously.1–4 In brief, it is likely that key outcomes for Oceania were underestimated, as the capacity to collect reliable data on haematological malignancies is often lacking in low-SDI regions. However, this does not considerably change our findings of disparities in disease burden and temporal trends observed between Australasia and Oceania, as well as between male/female sex. Moreover, data variation among the included countries in both regions, such as data quality, accuracy and the degree of missing data, might contribute to the deviation in the estimates, leading to discrepancies between regions.1 Second, GBD data include disease classifications by sex and age groups, but lacks ethnicity data, which limits the analysis of genetic susceptibility to disease. Third, a direct comparison between Australasia and Oceania could be difficult as there are considerable differences in the population distribution and risk factors between the two regions.1–4 As such, to explore the potential impacts attributed to age and sex distribution, our analyses used both region-specific populations (base-case) and standard population weights (sensitivity analyses) to explore changes in age-standardised outcomes. Fourth, it was not possible to discern the impact of various exposures on disease outcomes using aggregate data; as such, trends should be interpreted with caution due to potential confounding.1–4 Additionally, our EAPC model were limited to detect constant linear trends during the 10 year period. Future research could consider capturing non-linear trends during this explored period. Moreover, trends of leukaemia/lymphoma burden were predicted based on a single measure, ASR, for all age groups. This is likely to overlook the differences in trends between children/adolescents and adult population, who might have distinct characteristics and exposures to leukaemias/lymphomas. Finally, it was not possible to capture the impacts of the COVID-19 pandemic on leukaemia/lymphoma burden using GBD 2019 data.2 3 34
Ultimately, our findings highlight disparities in the management and treatment of haematological malignancies based on SDI and sex. Despite the emergence of novel therapies and improved treatment/management over time, the consistent and considerable burden of AML and NHL in both regions warrants further research to mitigate the gap attributed to socioeconomic disadvantage. Further research is also recommended to explore factors that contribute to disparate outcomes for sex (male/female).
Conclusion
This study captures the disease burden of leukaemias/lymphomas and its temporal trends in two closely situated geographic regions with different SDI. The considerable disparity in disease burden observed between the two regions suggests the need for early diagnosis and better management strategies tailored to each region. Further study is required to explore the underlying factors behind the epidemiological trends of different leukaemia and lymphoma subtypes in each region and the male predominance in the majority of leukaemia and lymphoma burden of disease.
Data availability statement
All data relevant to the study are included in the article and uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
TQAH and PL are joint first authors.
Contributors HTQA and LP—conceptualisation, data collection, formal analysis, investigation, methodology, visualisation, writing—original draft, and writing-review and editing. GL—conceptualisation, investigation, methodology, supervision, writing-review and editing. GL is the guarantor. Chat GPT was used to improve language and grammar.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.




