Article Text
Abstract
Objectives Randomised trials for the management of drug-resistant infections are challenging to conduct as target patient populations often lack decision-making capacity, and enrolment windows are typically short. Improving informed consent and risk communication in these trials is especially crucial for protecting patient interests and maximising trial efficiency. This study aimed to understand challenges in risk communication and informed consent in antimicrobial clinical trials.
Design Scoping review.
Data sources Searches were conducted in Embase, Medline, CINAHL and Web of Science Core for peer-reviewed English articles that were published from January 2000 to April 2023.
Eligibility criteria Included articles were empirical studies or expert opinions that sought experts’, patients’ or representatives’ opinions on informed consent in the context of clinical trials involving antibiotic/anti-infective agents.
Data extraction and synthesis Abstract screening, full-text review, data extraction and evidence rating were performed by two independent reviewers. Extracted data were summarised and reported qualitatively based on common themes. A total of 2330 records were retrieved, and 29 articles were included in the review.
Results Half of the articles involving medical experts and one-third involving patients and representatives reported that full comprehension by patients and representatives was challenging or not achievable. Healthcare providers and consent takers were crucial for the quality of informed consent. The level of trust consent givers placed on healthcare providers had a critical influence on the consent rate. Emotional distress was pervasive among patients/representatives.
Conclusion The findings indicate that strengthening consent takers’ communication skills in providing emotional support to patients and their representatives may improve informed consent. More research is needed to understand informed consent in low-income and middle-income and non-English-speaking countries.
- clinical trial
- medical ethics
- patient participation
- systematic review
Data availability statement
Data are available on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study includes views from experts and patients or representatives on informed consent.
This study advances the understanding of challenges in informed consent in antimicrobial trials.
The main limitation is that this study predominantly focuses on bacterial infections and thus has limited generalisability to other types of trials.
Introduction
Expensive and inefficient randomised trials for novel antibiotics and diagnostics are key factors contributing to the ‘valley of death’ for research and innovation in this field.1 This leads to delays in regulatory approvals for these life-saving drugs and deters pharmaceutical companies from investing in antimicrobial drug discovery.2 3 One contributing hurdle to inefficiency in these trials is low consent rates coupled with poor quality of informed consent.4–7 Poor quality of informed consent can harm the public’s trust in healthcare and medicine. Slow recruitment in clinical trials threatens internal validity by increasing the risk of confounding factors, differential attrition and operational drift, while it compromises generalisability by potentially altering the target population, reducing temporal relevance and introducing selection bias.8 9
Informed consent involves ‘voluntary authorisation, by a patient or research subject, with full comprehension of the risks involved’10 and is one fundamental ethical requirement for human subject research. Risk and uncertainty exist when information is incomplete, and our knowledge of the negative outcomes, benefits or other aspects of a medical treatment is limited during the informed consent procedure.11–13 In most medical research, risk usually refers to the possibility of having undesirable outcomes such as adverse effects. Poor communication of the trial information is one of the main reasons for the ineffective informed consent.8
Treatment strategy trials for multidrug-resistant infections hold unique challenges for informed consent. These challenges include strict enrolment criteria, limited time frame for enrolment, and target patient populations not having decision-making capacity for consent due to underlying severe infections. Specifically, the window for recruitment and consent is often narrow as the antibiotics under evaluation need to be administered as quickly as possible to control infections.
These challenges are exacerbated by other pervasive reasons behind poor understanding of informed consent forms and low consent rates for other types of clinical trials. Several studies found that information sheets, including templates provided by institutional research boards, are difficult to read,14 15 have great variability or insufficient explanation when stating risks and/or benefits16 17 and might not encourage decisions that meet recommendations such as the International Patient Decision Aids Standards instrument.6 The issue might be exacerbated by language and literacy barriers, especially those in low-income to middle-income countries.18 Second, doctor–patient communication is often inadequate in explaining complex concepts such as randomisation, placebo and priority given to patient well-being.4 19 While several strategies such as improving doctor–patient communication and relationships have been implemented to optimise recruitment in clinical trials, there is a lack of evidence-based strategy.8 Despite the introduction of ‘good clinical practice’ guidelines by the WHO,5 20 systematic reviews show that participants’ understanding of clinical trials, especially risk and side effects, had no substantial improvement over the past two decades.
There is a need for evidence-based strategies which balance individual patient autonomy and broader societal justice derived from successfully completed clinical trials. The current review aimed to understand the challenges in informed consent in the context of antimicrobial trials, by focusing on issues around risk communication, including patients’ concerns about the risk and uncertainty from experts’ and consent givers’ perspectives. We sourced both empirical studies that address patients’ perspectives and articles that present domain experts’ views. The specific objectives are to ascertain: (1) experts’ views and recommendations on risk communication; (2) patients’ or representatives’ concerns around risk and uncertainty when deciding on participation and (3) how communication of trial information and other factors could influence consent in the context of antimicrobial clinical trials.
Methods
Search strategy
We conducted searches in the following databases: Embase via Elsevier, Medline via Elsevier, PsycINFO via Ovid, CINAHL via EBSCOhost and Web of Science Core. The initial searches were conducted on 26 December 2022, and update searches were conducted on 26 April 2023. The search strategy aimed to locate peer-reviewed articles published in the English language from January 2000 for relevance and recency considerations in relation to treatment approaches and regulatory aspects. The details about the searches and full-search strategies are found in online supplemental material. All results were collated using both the SR-accelerator21 and EndNote.
Supplemental material
Data selection
The inclusion criteria were (1) in the context of clinical trials involving antibiotic/anti-infective agents; (2) empirical studies (eg, qualitative or quantitative) or an expert opinion guideline (experts defined in this review included health professionals, academics or researchers, research staff and regulators) and (3) addressed one or more of the following topics: patients’ willingness to participate in trials; risk and benefit considerations when participating in trials; content of informed consent; ethical issues relating to informed consent. The exclusion criteria were (1) studies that tested the efficacy or safety of a drug; (2) focused on antibiotic prescription in healthcare settings or (3) articles that emphasised cases (eg, vaccines, parasites, HIV or tuberculosis) that have more unique treatment approaches and regulatory considerations, and patients are typically less acutely unwell or a decision for treatment was less urgent. Title and abstract screening and full-text screening were performed by two reviewers (YS and AS-RP). Discrepancies in selecting the final included studies were resolved by consensus or a third reviewer (YM). Data selection was performed using SR accelerator and COVIDENCE.22
The quality of evidence from each shortlisted study was rated by two reviewers (YS and JEY) based on the modified Oxford Centre for Evidence-Based Medicine (OCEBM) levels of evidence. Level 1 referred to the highest level of quality (including randomised controlled trials with proper power) while level 5 referred to the lowest level of evidence (including case reports and opinions).23
Data extraction
Data extracted included the country/countries where the study was conducted, the type of clinical trial, and the target patient population. Data extracted for empirical studies also included study sample details (sample size and sample characteristics), methods (survey, interview and focus groups), and results and themes relating to informed consent. Data extracted from experts’ articles included opinions and statements in relation to consent. Initial data extraction was performed by two independent reviewers (any two of JEY, AS-RP and YS). The aggregated data were then reviewed and revised by all reviewers (JEY, AS-RP and YS). The extracted qualitative data were synthesised in a narrative format and categorised based on common themes by YS and were revised by JEY. All authors reviewed the final themes.
Patient and public involvement
None.
Results
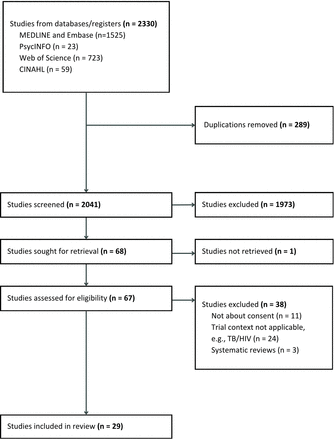
A total of 2041 unique records were screened and assessed by two independent reviewers. A total of 29 articles were selected for data extraction. These included 14 experts’ opinions, 11 studies that focused on the views of patients or representatives and 4 included both expert and patient responses (see figure 1). Three, 1, 11 and 14 articles were from OCEBM levels 1, 3, 4 and 5 evidence, respectively.
PRISMA flow chart of evidence selection. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; TB, tuberculosis.
Among the 18 articles based on experts’ views (12 articles by individual experts and 6 articles summarising aggregated experts’ views), the vast majority of the experts were doctors or medical researchers in English-speaking high-income countries such as the USA, the UK, Canada and Australia (17/18, 94%) (table 1). Three articles focused on informed consent for minors, two for pregnant women, one for older adults and one for participants in developing countries. Among the 15 articles based on patients’ and representatives’ views, 5 focused on minors, 2 on pregnant women and 1 on older adults (see table 2).
Characteristics of included papers synthesising expert views
Characteristics of included papers synthesising views of patients and representatives
Achieving informed consent is challenging
A frequent concern among experts was that true informed consent with full comprehension by patients and representatives was challenging or not achievable24–31 (table 3). One reason was that because clinical trials are meant to establish evidence or explore uncertainties for the interventions they are testing, specific risks may not be clearly known at the time of research.24 27 32–34 Other reasons included patients and representatives being unable to fully understand the research,25 31 35 due to a lack of health literacy, complexity of research terms, and cultural and language barriers. While improving patients’ understanding28 29 36 37 was frequently recommended for improving informed consent, experts were also concerned that patients might have cognitive impairment or declined cognitive capacity in acute illness, who might be deemed to have decision-making capacity but unable to fully comprehend the complexities of the proposed research.26 27 29 35
Summary of main findings
On the other hand, patients and representatives valued being well-informed and receiving information about the research.25 35 38–40 However, recurrent themes included the difficulty, lack of or misunderstanding of research and trial designs, especially randomisation and blinding.39–43 Patients had an inaccurate understanding and underestimated the risk of the research.41 42 44 45 Patients believed that there was minimal or even no risk involved in the research44 while overestimating the benefit or being overoptimistic about the treatment.41
Doctors and research staff are critical for the success and quality of consent
The experts generally agreed that doctors and research staff hold the responsibility to explain risks to patients.24 27 33 37 However, doctors’ and research staff’s own preferences, understanding, and experiences might influence risk communication with patients and patients’ consent.25 35 43 Corneli et al 25 reported that the doctors and research staff might have misconceptions about terms like non-inferiority, and their misunderstanding could negatively impact their risk communication with patients. Similarly, staff or doctors-related factors were the most commonly raised39–44 46 by patients and representatives. Those factors included trust in doctors and research staff,39 41 42 44 46 doctors’ attitudes and opinions and how they frame risks during the communication,39 41–44 46 and friendliness40 and sympathy39 42 from the staff. Furthermore, the need for counselling or discussion between patients and representatives and doctors and staff, including exploring alternative options39 43 was proposed by patients, representatives and experts.24 27 28 43 47 Providing training to doctors and staff29 36 43 was recommended for improving informed consent.
Consent forms
Several articles mentioned informed consent forms having either too much information, insufficient details for participants to understand the research or being prone to misinterpretation by participants.35 44 45 48 Three articles investigated the effect of the format and framing of information sheets on participants’ perceptions or consent.45 48 49 The framing of the side effects might influence risk perceptions when participants spent adequate time reading the information but did not appear to influence consent or perceived research credibility.45
Patients’ concerns centred around risks and benefits to individual and wider population
Experts recognised a range of factors that influence patients' decision to provide informed consent, especially those relating to trial properties and outcomes such as the study’s risk and benefit,31 32 altruism,31 32 convenience (eg, logistics, flexibility in time),27 36 financial hurdles32 and social interaction with others and partnership (eg, patients’ expertise, trust and contribution are acknowledged) during the trial participation.32 Similar factors were mentioned by patients and representatives, including health-related risk and outcomes,35 39 40 42 44 49 perceived benefit to the patient’s health condition and hope,35 39 41 42 44 altruism (eg, benefiting science and medical research, and other patients),35 40–42 44 50 logistics and opportunity cost,39 46 incentives and cost incurred due to complications,40 49 and disruption to social lives.39 Patients and representatives were also motivated by their interest in the study35 49 and the belief that they might receive better care41 through trial participation.
Both experts and patients also indicated trust as an important factor, including patients’ trust in medicine,28 36 the system and government regulation,39 40 42 44 and science and medical research.40 44 Patients’ rights to withdrawal, autonomy (eg, being able to make a choice or act based on their will) and having had a decision or preference for a specific treatment option were also frequently mentioned.40 46 49 50
Consent procedures can be time-constrained and distressing
Experts expressed that the consent taking procedures, especially complex ones, can be laborious and increase the workload of healthcare professionals.29 31 33 35 38 While experts recommended allowing more time for consent givers to make decisions,27 28 43 time-related issues such as time pressure were experienced by both experts and consent givers.42–44 Recruiting doctors might face the challenge of time constraints during the usual doctor consultation.31 Meanwhile, consent givers reported that they relied on common sense and heuristics during decision-making44 and might have had little consideration during the process.43 44
It was also observed that negative emotions, especially emotional distress, during the decision process among patients and representatives were reported in almost all the primary research studies.38–44 Anxiety, fear, and worry were the common emotions expressed or shown by patients and representatives. Relating to the consent takers factors above, patients appreciate empathy from recruiting staff.39 42
Alternatives to conventional consenting process
Experts expressed concern that conventional informed consent after infection onset can be impractical.26 27 29 51 Some experts suggested the implementation of advanced consent and early enrolment (consent and enrolment before a patient becomes eligible for a study) prior to infection onset.29 35 38 Patients and relatives also expressed no major concerns about early recruitment/enrolment or advanced consent.35 38
Discussion
The current review explored challenges in informed consent by focusing on risk communication, including patients’ concerns about risk and uncertainty, in the context of antimicrobial trials. One key finding in our review was that achieving true informed consent can be challenging. Doctors and research staff were suggested to be the most essential in the informed consent and risk communication process. Trust in doctors and staff, medical research, and the healthcare and regulatory systems were key influences during consent givers’ decision-making. Lastly, there was pervasive emotional distress among patients and representatives during the consent procedure.
The finding that true informed consent might not be achieved, either due to the lack of understanding or the lack of capacity from patients and representatives, aligned with previous systematic reviews that consent givers’ misunderstanding of clinical trials was one of the main issues in informed consent.5 20 Given that clinical research is difficult to explain, patients’ trust in doctors and research becomes critical for informed consent. The role of trust in patient decisions is also discussed in the previous literature.4 52 Believing that doctors and staff have their best interests and that safety is ensured via strict regulation reassures consent givers that any risks or negative consequences will be managed and minimised. However, trust could also be a double-edged blade, especially when consent givers do not have an accurate understanding of the research. Doctors and research staff may consciously or unconsciously express their own preferences and biases when communicating with consent givers and sometimes may even have misconceptions about the research. These, in turn, influence consent givers’ understanding and decisions. Consent givers might also overly rely on trust rather than engaging in understanding the research. The experience of adverse effects that were not expected by patients due to misunderstanding can result in substantial damage to their trust in medicine.28 44
Furthermore, we observed that consent givers, including patients and family members, expressed anxiety, fear, worry, and feeling overwhelmed during the decision process. This is in line with the observation by a previous study that found that anxiety associated with these high-stakes interventions may impact patients’ ability to understand the documents and make informed decisions about participation in the trial.15 Anxiety and fear can influence risk and benefit perceptions, thus influencing informed decision.53 54 Managing consent givers’ negative emotions and showing empathy and sensitivity by staff can be important during the informed consent procedure.
Our review did not find evidence that informed consent forms played a crucial role in consent for antimicrobial clinical trials. In fact, many participants might spend little time reading the information sheets in hypothetical clinical trials.49 Consent givers in real trial settings might feel having little time to process the given information, and thus may largely rely on heuristics.55–57 Although it has been recommended that sufficient time should be allowed for consent givers to understand the information and make decisions,27 28 43 time constraints can still be challenging, especially in trials with narrow recruitment windows. An alternative solution is allowing advanced consent and early enrolment (ie, before patients become eligible), to address issues including patients having limited decision time or lack of decision capacity, which were found acceptable by both experts and patients or their representatives.
We found a lack of research on informed consent in antimicrobial resistance trials in low-income to middle-income countries. This contrasts with a review by the US Food and Drug Administration, which included 42 phase 3 antibiotic trials that showed just 16.7% of participants were from the USA.58 A recent systematic review found that the consent rate in low-income to middle-income countries was significantly higher than in high-income countries.59 However, the quality of the informed consent might be questionable as language and cultural barriers in developing countries might exacerbate the comprehension issues in informed consent.60–63 Participants’ consent in developing countries might also be influenced by unique factors such as social influence,60 free medical care and opportunities to gain knowledge and skills during the trial participation.61 62 Meanwhile, significant disparities exist where middle-income and lower-middle-income countries have limited access to healthcare including antibodies.64 Risks and benefits of trials and participants’ motivations to consent in middle-income and lower-middle-income countries encompass a unique set of ethical challenges.65 It is critical to understand informed consent from participants in low-income to middle-income countries.
Several limitations of this review should be noted. First, we included articles which predominantly focused on bacterial infections. However, our findings may be extrapolated to other medical conditions and clinical trials which are time-sensitive. Second, we focused on risk and uncertainty communication during informed consent. Future research may have broader investigations on other factors that may influence informed consent. Furthermore, challenges in recruitment and issues of trial validity go beyond those in risk communication, comprehension, and acceptance of trial participation. The extent to which a trial is inclusive in reaching patients from diverse backgrounds also influences the trial recruitment and generalisability of the trial results. Inclusiveness and diversity have been increasingly emphasised by both scientific communities and regulatory bodies.66 Future research should have a more in-depth understanding of the interplay between consent, inclusiveness, and diversity in trial conduct.
Finally, the articles in the current review are exclusive academic articles and have been more focused on issues relating to consent givers. Successful recruitment, effective risk communications and high-quality conduct of trials can depend on investigators’ ability to conduct trials and the availability of the research staff to invest in the time to facilitate consent. Future research should also include challenges relating to trial investigators and regulators (eg, institutional review boards) and review literature beyond traditional academic publications.
In conclusion, our review found that difficulty in achieving full informed consent and adequate comprehension among patients and representatives, exacerbated by a narrow consent window, are the major challenges in antimicrobial trials. Improving professionality, communication skills and empathy among doctors and staff may improve consent quality, reduce negative emotions associated with the consent procedure, and promote trust building. Table 4 summarises the main recommendations for improving informed consent and consent rate based on the articles and current review. Meanwhile, more research and empirical evidence are needed to develop more systematic and effective guidance for those recommendations. The current review also highlights the knowledge gap in developing countries and non-English-speaking populations and calls for more research in under-researched populations.
Recommendations for improving informed consent and consent rate
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors YS, YM and DLP conceptualised and designed the study. YS, JEY and AS-RP contributed to data collection. YS wrote the original draft and acted as guarantor. All authors contributed to interpretation and reviewed, edited and approved the final version of the manuscript.
Funding This research is supported by a National University of Singapore Start-Up Grant (award/grant number is not applicable) and a Wellcome Trust Grant (Ref 227155/Z/23/Z).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Author note This study was preregistered at https://osf.io/fu49y/. We report OCEBM levels of evidence as quality appraisal ratings in this manuscript instead of JBI/CASP as preregistered due to the significant heterogeneity in the articles included in this review.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.