Article Text
Abstract
Objective This post hoc analysis compared the immunogenicity of the biosimilar adalimumab-adbm (Cyltezo) with the adalimumab reference product (RP; Humira) across indications, including rheumatoid arthritis (RA), Crohn’s disease (CD) and plaque psoriasis (PsO), and by patient sex in the VOLTAIRE trials programme.
Methods In each active-comparator randomised controlled trial (RCT), immunogenicity was assessed at various time points by the proportion of patients with antidrug antibodies (ADAs) and neutralising antibodies (nAbs), using acid dissociation followed by electrochemiluminescence assay. Assay sensitivity was 50 ng/mL, and drug tolerance was ≥30 µg/mL (free drug) at the low positive control level.
Results Minor differences in immunogenicity parameters (ADAs, ADA titres and nAbs) were evident between adalimumab-adbm and adalimumab RP across these three immune-mediated inflammatory diseases (IMIDs). The proportion of ADA-positive and nAb-positive patients increased from baseline over time in all three RCTs, as expected, and was similar in the RA and CD RCTs but with higher numbers of ADA-positive and nAb-positive patients reported in the PsO trial. Subgroup analysis by patient sex showed the same trend.
Conclusions Differences among the RCTs may partially be explained by concomitant background therapy (methotrexate) in the RA trial, stable doses of azathioprine, 6-mercaptopurine or methotrexate in 36% of patients with CD and absence of background therapy in the PsO RCT. The analyses further confirm the biosimilarity of adalimumab-adbm with the adalimumab RP across IMIDs and provide supporting evidence that adalimumab-adbm is an interchangeable biosimilar with consistent clinical results in patients originally treated with the RP.
Trial registration numbers VOLTAIRE-RA (NCT02137226; EudraCT 2012-002945-40); VOLTAIRE-CD (NCT02871635; EudraCT 2016-000612-14); VOLTAIRE-PsO (NCT02850965; EudraCT 2016-000613-79).
- rheumatology
- inflammatory bowel disease
- chronic disease
- psoriasis
Data availability statement
To ensure independent interpretation of clinical study results and enable authors to fulfil their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer-reviewed journal, providing regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study used a fully validated single-bridging electrochemiluminescence assay (ECLIA) following acid dissociation to measure the presence and titre of antidrug antibodies (ADAs).
ECLIA is not only simpler and faster than ELISA but also provides more sensitive and precise results. ECLIA has a far higher drug tolerance than ELISA, allowing ADA levels to be detected at any time point in the VOLTAIRE trials.
While the same treatments and analysis methods were used across the different VOLTAIRE trials, there were important differences between the background medications and patient populations that were included in each trial.
Comparisons to immunogenicity data from previous trials may be complicated by the increased sensitivity and stringency demonstrated by current immunogenicity assays and by the different visit schedules among the trials.
Introduction
The monoclonal antibody (mAb) adalimumab-adbm (Cyltezo, Boehringer Ingelheim) is approved in the USA as an interchangeable biosimilar to the adalimumab reference product (RP; Humira, AbbVie).1–3 Adalimumab-adbm and adalimumab RP have structural similarity, comparable activity in blocking tumour necrosis factor (TNF), and are pharmacokinetically bioequivalent.4 5 The phase III VOLTAIRE clinical development programme demonstrated that the efficacy of adalimumab-adbm was equivalent to adalimumab RP with comparable safety and immunogenicity in patients with moderate to severely active rheumatoid arthritis (VOLTAIRE-RA), moderate to severely active Crohn’s disease (VOLTAIRE-CD) or moderate-to-severe chronic plaque psoriasis (VOLTAIRE-PsO).6–8 In VOLTAIRE-RA and VOLTAIRE-CD, treatment benefits were maintained in patients who received adalimumab RP and were switched to adalimumab-adbm.6 7
TNF inhibitor (TNFi) treatment is unsuccessful in up to 40% of patients according to European League Against Rheumatism response criteria, either because of failure to attain any response or loss of response over time.9 One reason for loss of response is the development of antidrug antibodies (ADAs), as these newly formed immune complexes are biologically less active or cleared more readily by the kidneys.10 11 In essence, ADAs lower the effective dose of therapeutic mAb by limiting its bioavailability in the circulation. The presence of ADAs not only results in subtherapeutic serum drug concentrations and treatment resistance but also leads to adverse effects such as infusion reactions/anaphylaxis, drug-induced lupus and vasculitis-like events.12–14
The incidence of ADAs to TNFi varies between mAbs due to factors such as structure, extent of humanisation and route of administration and between trials for various reasons, including patient-related factors (eg, genetic predisposition, concomitant medication use), disease activity, treatment-related factors (eg, sampling timing, treatment duration) and the ADA detection methodology used (eg, type of assay and drug trough levels).9 14–17 Detecting and characterising ADAs with respect to serum titre and neutralising capacity are key to understanding their relationship with safety and efficacy outcomes.18 ELISAs are often used for ADA screening, but their utility is limited by a low drug tolerance—the maximal amount of free drug in the sample that still results in a detectable ADA signal—and their capacity to detect only free ADAs.
In VOLTAIRE, a fully validated single-bridging electrochemiluminescence assay (ECLIA) following acid dissociation was used to measure the presence of ADAs and their titres.5 ECLIA is not only simpler and faster than ELISA but also provides more sensitive and precise results.19 Importantly, ECLIA has a far higher drug tolerance than ELISA, allowing ADA levels to be detected at any time point in the VOLTAIRE trials.5 The aim of this post hoc analysis was to compare the immunogenicity of adalimumab-adbm and adalimumab RP in VOLTAIRE-RA, VOLTAIRE-CD and VOLTAIRE-PsO. ADAs and neutralising antibodies (nAbs) were assessed to identify at what point they were first detectable, determine the proportion of patients with ADAs and nAbs over time and explore any observed effects on clinical responses.
Methods
Study designs and participants
The design, methods and conduct of the phase III VOLTAIRE-RA, VOLTAIRE-CD and VOLTAIRE-PsO randomised controlled trials (RCTs) have been described previously and are summarised in table 1.6–8 In addition to the different patient populations, although prior adalimumab use was an exclusion criterion in all three trials, there are important differences between the background medications given in each trial. All patients in VOLTAIRE-RA had to have received stable background methotrexate therapy for ≥12 weeks prior to enrolment, which was not used in VOLTAIRE-CD or VOLTAIRE-PsO.6–8 Patients in VOLTAIRE-CD were either naive to TNFi therapy or had previously been treated with infliximab and developed secondary resistance due to anti-infliximab ADA formation or had become intolerant.7 Patients in VOLTAIRE-CD and VOLTAIRE-PsO received a loading dose of either adalimumab-adbm or adalimumab RP, whereas VOLTAIRE-RA patients were initiated on 40 mg every 2 weeks (ie, the maintenance dose in all three trials). Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of the research.
Features and properties of the multicentre, active-comparator VOLTAIRE randomised controlled trials
Assessments
Immunogenicity
Fully validated assays for the detection and quantification of ADAs and nAbs have been described elsewhere.5 In brief, ADA positivity and titres were determined using acid dissociation followed by single-bridging ECLIA (MSD platform; Meso Scale Diagnostics, USA).5 Acid dissociation followed by ECLIA for detection of ADAs is independent of serum drug concentrations. In VOLTAIRE, assay sensitivity was 50 ng/mL and drug tolerance was ≥30 µg/mL (free drug) at the low positive control level. The presence of nAbs was determined using a cell-based antibody-dependent cell-mediated cytotoxicity method with a sensitivity of 1.5 µg/mL.
Efficacy
The primary endpoints that were prospectively evaluated in VOLTAIRE-RA, VOLTAIRE-CD and VOLTAIRE-PsO were American College of Rheumatology 20% response criteria (ACR20), Crohn’s Disease Activity Index (CDAI) decrease ≥70 points and 75% reduction in Psoriasis Area and Severity Index (PASI 75), respectively.6–8 In VOLTAIRE-RA, coprimary endpoints were the percentage of patients with ACR20 responses at weeks 12 and 24.6 The percentage of patients with CDAI scores ≥70 points at week 4 was the primary endpoint in VOLTAIRE-CD7 and the percentage of patients with PASI 75 responses at week 16 was the primary endpoint in VOLTAIRE-PsO.8
Statistics
All patients who received ≥1 dose of adalimumab-adbm or adalimumab RP in the VOLTAIRE clinical trials were included in the immunogenicity analysis (safety analysis set). Immunogenicity was assessed as the proportion of patients with ADAs and/or nAbs at baseline and prespecified time points post dose (table 1). Patient-level immunogenicity data were analysed separately in each VOLTAIRE RCT and by sex. Differences in study population and methodology precluded formal comparisons between trials. Clinical responses based on the primary efficacy outcome for each RCT were reported according to ADA and nAb positivity. Non-responder imputation was used for patients who discontinued prior to that time point and last observation carried forward for patients with missing data.
Continuous and categorical characteristics were summarised descriptively by treatment and by
disease indication, with categorical data summarised as N (%), normal continuous data as mean (SD) and skewed continuous data as median (Q1, Q3). Incidence rates were calculated by disease indication and by treatment arm. Kaplan-Meier estimates will be used to display the distribution of time to first event for each treatment group by disease indication with 95% CIs found using Greenwood’s variance estimate.
All data analyses were performed using descriptive statistics compiled by SAS software (SAS, Cary, North Carolina, USA; V.9.4 or higher).
Results
Study populations
The analysis populations are described in table 2. The average age of patients in the VOLTAIRE-RA population was around 10 years older than the VOLTAIRE-PsO population, which in turn was 5–10 years older than the average age of patients in the VOLTAIRE-CD population. More than three-quarters of patients (83.1%) in VOLTAIRE-RA were women, whereas women comprised less than half of the population in VOLTAIRE-CD (43.5%) and VOLTAIRE-PsO (36.0%). Across all VOLTAIRE trials, >95% were White patients.
Patient demographics and clinical characteristics at baseline in VOLTAIRE-RA, VOLTAIRE-CD and VOLTAIRE-PsO RCTs (safety analysis sets)
A significant minority of patients in VOLTAIRE-RA and VOLTAIRE-PsO trials had previously received a biologic agent (19%–27%) compared with <10% of VOLTAIRE-CD patients. In VOLTAIRE-RA, the mean (SD) dosage of background methotrexate at baseline was 16.3 (3.6) mg/week in the adalimumab-adbm arm and 16.8 (3.9) mg/week in the adalimumab RP arm. In VOLTAIRE-CD, stable doses of azathioprine, 6-mercaptopurine or methotrexate were received by 36% of patients.
Immunogenicity
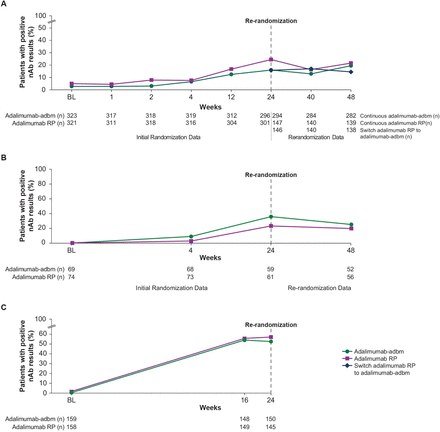
Although patients in all three trials were excluded for prior adalimumab use, positive ADA titres were detected at baseline in all VOLTAIRE treatment arms except the adalimumab RP group in VOLTAIRE-CD (table 3). ADA titres increased over time in all VOLTAIRE treatment arms, with evidence of a plateau at week 24 in VOLTAIRE-RA and VOLTAIRE-CD (figure 1). Incidence of ADAs was lowest in VOLTAIRE-RA and highest in VOLTAIRE-PsO. In VOLTAIRE-RA, incidence of ADAs was similar in the adalimumab-adbm and adalimumab RP arms to week 24 and after rerandomisation to week 48. Incidence of ADAs was also similar in both treatment arms in VOLTAIRE-PsO, but higher in the adalimumab-adbm arm than the adalimumab RP arm of VOLTAIRE-CD.
Time course of ADA development in (A) VOLTAIRE-RA, (B) VOLTAIRE-CD and (C) VOLTAIRE-PsO (safety analysis sets). ADA, antidrug antibody; CD, Crohn’s disease; PsO, plaque psoriasis; RA, rheumatoid arthritis; RP, reference product.
Immunogenicity in the phase III VOLTAIRE RCTs6–8
nAbs were detected at baseline in both treatment arms of VOLTAIRE-RA and VOLTAIRE-PsO and at week 4 in both treatment arms of VOLTAIRE-CD (table 3). The trajectory of nAb incidence was similar to that of ADA incidence (figure 2). nAb development was lowest in VOLTAIRE-RA and VOLTAIRE-CD and highest in VOLTAIRE-PsO. nAb development was similar in the adalimumab-adbm and adalimumab RP arms at all time points in VOLTAIRE-RA and VOLTAIRE-PsO and appeared higher in the adalimumab-adbm arm in VOLTAIRE-CD.
Time course of nAb development in (A) VOLTAIRE-RA, (B) VOLTAIRE-CD and (C) VOLTAIRE-PsO RCTs (safety analysis sets). CD, Crohn’s disease; nAb, neutralising antibody; PsO, plaque psoriasis; RA, rheumatoid arthritis; RCT, randomised controlled trial; RP, reference product.
Across treatment arms in VOLTAIRE-RA and VOLTAIRE-PsO, attainment of the primary efficacy endpoint was less frequent among patients with positive ADA and nAb titres (figures 3 and 4), although ADA and nAb development appeared to have no effect on the primary endpoint in VOLTAIRE-CD. There was no difference between adalimumab-adbm and adalimumab RP with respect to the primary endpoints in VOLTAIRE-PsO, and efficacy outcomes were not affected by positive ADA or nAb titres.
Frequency of patients meeting primary clinical response criteria per treatment arm stratified by ADA titre positivity (safety analysis sets) for VOLTAIRE-RA, VOLTAIRE-CD and VOLTAIRE-PsO RCTs. ACR20, American College of Rheumatology 20% response criteria; ADA, antidrug antibody; CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; PASI, Psoriasis Area and Severity Index; PASI 50 / PASI 75, 50% or 75% reduction in PASI, respectively; PsO, plaque psoriasis; RA, rheumatoid arthritis; RCT, randomised controlled trial; RP, reference product.
Frequency of patients meeting primary clinical response criteria per treatment arm stratified by nAb titre positivity (safety analysis sets) for VOLTAIRE-RA, VOLTAIRE-CD and VOLTAIRE-PsO RCTs. ACR20, American College of Rheumatology 20% response criteria; CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; nAb, neutralising antibody; PASI, Psoriasis Area and Severity Index; PASI 50 / PASI 75, 50% or 75% reduction in PASI, respectively; RCT, randomised controlled trial; RA, rheumatoid arthritis; RCT, randomised controlled trial; RP, reference product.
The proportion of ADA-positive and nAb-positive patients at baseline and throughout in VOLTAIRE-RA and VOLTAIRE-PsO RCTs did not differ by patient sex (online supplemental tables S1–S3). In contrast, in VOLTAIRE-CD approximately two times as many women than men developed ADA-positive and nAb-positive titres in the adalimumab RP arm, though this difference was not seen in the adalimumab-adbm arm.
Supplemental material
Discussion
The immunogenicity data for adalimumab-adbm has been well characterised across the VOLTAIRE trials, providing information on the experience with adalimumab-adbm relative to adalimumab RP across different immune-mediated inflammatory diseases and by sex. These analyses confirm the biosimilarity of adalimumab-adbm with adalimumab RP in adult patients with RA, CD and PsO. ADA and nAb development were similar in the two treatment arms in VOLTAIRE-RA and VOLTAIRE-PsO, appearing higher in the adalimumab-adbm arm of VOLTAIRE-CD. However, the presence of ADAs and nAbs had no bearing on the primary efficacy outcomes in any of the trials.
Treatments, sample timings and analysis methods were consistent across the different VOLTAIRE trials. However, there remain important differences between the patient populations and consequentially, in the background medications and that were included in each trial. Patients with RA and CD regularly receive glucocorticoid prescriptions, both oral and parenteral. However, glucocorticoids do not abrogate or decrease ADA responses; agents that do mitigate these responses are antiproliferative agents, specifically methotrexate, azathioprine, leflunomide and mycophenolate mofetil, if given with the first dose of the biologic therapy. Patients in the VOLTAITRE-RA trial received methotrexate as background therapy, while stable doses of azathioprine, 6-mercaptopurine or methotrexate were being given in 36% of patients in VOLTAIRE-CD, contrasting with an absence of these background therapies in VOLTAIRE-PsO. Higher rates of ADA development occurred in VOLTAIRE-PsO than VOLTAIRE-RA and VOLTAIRE-CD, likely owing to this lack of background therapy in the VOLTAIRE-PsO trial. ADAs and nAb titres after exposure to adalimumab-adbm and adalimumab RP were comparable in male and female patients across the VOLTAIRE trials, aligning with results of a multicentre retrospective cohort study.20 In addition to limitations resulting from differences in visit schedules, making any comparisons to immunogenicity data from historical RP is complicated by the increased sensitivity and stringency demonstrated by current immunogenicity assays used for biosimilars.21 Acid dissociation followed by the more sensitive ECLIA for detection of ADAs, as used in this study programme, is not dependent on serum drug concentrations. The positive ADA titres detected at baseline may have been due to false positives. While some patients had been exposed to other biologics prior to these studies, previous immunogenicity studies have shown no indication of cross-reactivity to other biologic agents including other TNF inhibitors.21 Finally, it should be noted that the data presented here are from patients with PsO, RA or CD and may not be applicable to other indications (eg, ankylosing spondylitis) for which adalimumab-adbm has also been approved.1
ADA titres, ADAs and nAbs were first measurable at day 1 in small numbers and increased over time. Immunogenicity parameters were similar between the RP and biosimilar throughout the period covered in each RCT, and the presence of ADAs or nAbs had no apparent effect on efficacy. These analyses further confirm the biosimilarity of adalimumab-adbm with adalimumab RP in adult patients with RA, CD and PsO.
Data availability statement
To ensure independent interpretation of clinical study results and enable authors to fulfil their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer-reviewed journal, providing regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants. The protocol was approved by the applicable independent ethics committee or institutional review board at each participating site (see individual study publications for details), 6–8 and the study was performed in accordance with Good Clinical Practice and the Declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
Writing support was provided by Malcolm Darkes on behalf of Envision Pharma Group and Andy Shepherd of Envision Pharma Group, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors All authors (VS, SB and DM) were fully responsible for the analysis and interpretation of the data, all content and editorial decisions, were involved at all stages of manuscript development and have approved the final version that reflects the authors’ interpretation and conclusions.
Funding This study was supported by Boehringer Ingelheim. The authors did not receive payment related to the development of the manuscript.
Competing interests VS reports consulting for AbbVie, Amgen, Aria, AstraZeneca, Bayer, Bioventus, BlackRock, BMS, Boehringer Ingelheim, Celltrion, ChemoCentryx, Equillium, Gilead, Genentech/Roche, Glenmark, GSK, Horizon, Inmedix, Janssen, Kiniksa, Kypha, Lilly, Merck, MiMedx, Novartis, Pfizer, Priovant, Regeneron, Rheos, R-Pharma, Samsung, Sandoz, Sanofi, Scipher, Setpoint, Sorrento, Spherix and Tonix. SB reports previous employment by Boehringer Ingelheim Pharmaceuticals. DM reports previous employment by Boehringer Ingelheim Pharmaceuticals.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.