Article Text
Abstract
Objectives Breast cancer is the leading cause of cancer morbidity and mortality among women. Still, there is a paucity of studies to know the magnitude of the problem in Ethiopia. Hence, this review was intended to pool the prevalence and identify the determinants of breast cancer in Ethiopia.
Design A systematic review and meta-analysis was conducted.
Data sources Databases like PubMed/MEDLINE, HINARI, Science Direct, and Google Scholar, as well as websites of organisationsI organizations,rewere searched between 25 February and 6 March 2023.
Eligibility criteria All observational studies in Ethiopia that reported either the magnitude and/or determinants of breast cancer regardless of publication status were included.
Data extraction and synthesis Two authors independently assessed and extracted the data. The Joanna Briggs Institute meta-analysis of statistics assessment and review instrument quality appraisal tool was used to assess the quality of the articles. Effect estimates were done by using the random-effects model. The meta-analysis results were displayed by using forest plots.
Results Seventeen articles were reviewed with 24 435 total participants. The pooled proportion of breast cancer morbidity among patients with cancer was 20. 58% (95% CI 17.25%, 23.90%) in Ethiopia. Consuming packed foods (POR=2.12, 95% CI 1.41, 3.17), presence of high cholesterol (POR=4.08; 95% CI 2.75, 6.07), physical inactivity (POR=3.27; 95% CI 1.80, 5.94), high body mass index (BMI) (POR=2.27; 95% CI 0.85, 6.03), postmenopause (POR=2.25; 95% CI 1.63, 3.10), family history of cancer (POR=3.65; 95% CI 0.85, 15.71) and lack of breastfeeding (POR=2.76; 95% CI 0.90, 7.92) were the determinants of breast cancer.
Conclusions One of five patients with cancer is diagnosed with breast cancer in Ethiopia. Furthermore, more than a quarter of women with cancer suffer from breast cancer. Processed food consumption, high cholesterol in the body, lack of physical activity, high BMI, postmenopause, family history of cancer and lack of breastfeeding were the risk factors for breast cancer. The use of healthy food sources such as fruits and vegetables, and homegrown varieties of crops rather than seeking processed foods would help.
PROSPERO registration number CRD42023417733
- Risk Factors
- Primary Health Care
- PUBLIC HEALTH
- Chronic Disease
Data availability statement
Data are available upon reasonable request. The data extracted from included studies and analysed in this review are available from the corresponding author upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The inclusion of prediction interval is a strong aspect of this study, as it is uncommon in many meta-analyses.
The review included only observational studies.
The narrow scope of the review (only one country) is a limitation of this study.
A limited number of studies were found to pool the OR for some factors.
Background
Breast cancer is a diverse disease with numerous morphological and molecular subgroups.1 It has been found to be the most common cause of cancer deaths in 11 regions of the world.2 It is one of the most frequently diagnosed cancers and the leading cause of cancer deaths in women worldwide.3 The recent global burden of cancer statistics (GLOBOCAN 2020) showed that breast cancer has surpassed lung cancer and accounted for 2.3 million (11.7%) of all new cancer cases globally. It affects one in four new cancer cases of women and contributes to one in six deaths of women from cancer.4
The cancer burden is increasing worldwide and is estimated to be 28.4 million cases by 2040, which is a 47% increase over the cancer burden in 2020. A higher death rate occurs in developing countries than in developed countries (15 breast cancer deaths in developing countries vs 12.8 in developed countries per 100 000).4 In Ethiopia too there were an estimated 5900 incident cases of breast cancer with the highest age-standardised incidence rate of 12.5 per 100 000 and a death rate of 9.7 per 100 000 in 2019.5
Previous studies identified that the incidence of breast cancer varies widely across the world due to differences in the level of education, economic status, environmental conditions, eating habits, lifestyle variables and other cultural traditions. Early age at menarche, westernised lifestyles (namely delayed pregnancies/childbirth, reduced breastfeeding, sedentary lifestyles and poor diet), and improving cancer registration and cancer detection are among the factors associated with the breast cancer in low and middle income countries (LMICs).6–9 Lack of knowledge about the disease, improper screening programmes, delayed diagnosis and insufficient medical facilities are also contributing factors to the increasing breast cancer burden in underdeveloped countries.6 10 11 Widespread urbanisation, shifting patterns of reproductive and environmental risk factors, obesity, decreased physical activity and rising life expectancy are among the major factors contributing to the steady rise in breast cancer incidence in low-income nations. Low socioeconomic level, on the other hand, is related to an increased incidence of aggressive premenopausal breast cancers, as well as late-stage diagnosis and lower survival. Late menopause and early menarche are also among the risk factors that could increase the exposure of breast tissue to oestrogen hormone. In contrast to this, pregnancy and appropriate breastfeeding help to reduce the risk of breast cancer.6 10–13
Comprehensive identification of the magnitude and determinants of breast cancer is critical for developing nations like Ethiopia, as this will aid in the development and implementation of effective breast cancer prevention initiatives. Breast cancer is not well studied in Ethiopia. Although there is one recently published review, the focus of that study was more on determinants of the problem.14 Different pocket studies so far may not represent the entire picture of the determinants of breast cancer in Ethiopia. Most of them were limited to small sample sizes, limited portions of populations covered and limited research regions. In this regard, many of the regions were not addressed in the previous studies and our study also helps to show this gap for further study, let alone intervention. It is critical to shed light on the risk factors for breast cancer. As a result, this study aimed to determine the magnitude of breast cancer and its determinants in Ethiopia.
Methods
Study design
A systematic review and meta-analysis was conducted. The protocol was registered on Prospective Registry of Systematic Reviews with registration number CRD42023417733 and no change was made to the protocol. To conduct this review, the Preferred Reporting Items for Systematic Reviews and Meta-Analysis checklist15 was used.
Search strategy
A comprehensive search of databases like PubMed/Medical Literature Analysis and Retrieval System Online (MEDLINE), Health Inter-Newtork Access to Research Initiative (HINARI), Science Direct and Google Scholar was used to find the relevant articles. The searches were limited to articles written using the English language. In addition to the electronic database search, grey literature was searched using Google search and digital libraries of universities. Finally, the reference lists of the included articles for related studies were searched. To facilitate the article searching process the following keywords were used: ‘breast’ OR ‘mammary gland’ AND ‘cancer’ OR ‘tumour’ OR ‘malignancy’ OR ‘breast cancer’ OR ‘breast malignancy’ OR ‘breast tumour’ AND ‘Risk factors’ OR ‘Associated factors’ OR ‘Determinants’ OR ‘predictors’ AND ‘Ethiopia’ OR ‘Addis Ababa’ OR ‘Northern Ethiopia’ OR ‘North west Ethiopia’ OR ‘Southern Ethiopia’ OR ‘South Western Ethiopia’ OR ‘Western Ethiopia’ OR ‘East Ethiopia’ (online supplemental additional file 1). Searching started on 25 February 2023 and the final date of searching was 6 March 2023.
Supplemental material
Supplemental material
Eligibility criteria
Inclusion criteria
To be included in this review, the study should report either the determinants of breast cancer and/or the magnitude (incidence, prevalence, number) of breast cancer morbidity.
Study setting
Studies conducted in Ethiopia (both institution-based and population-based) were part of this systematic review.
Study population
The study involved all the human population (male, female, children and adults) in Ethiopia who has been evaluated for cancer and confirmed to be patients with cancer.
Exposure
Those with modifiable or non-modifiable risk factors.
Study design
All observational studies (cross-sectional and case-control) that reported the magnitude of breast cancer morbidity and its determinants were evaluated to be included.
Publication status
Both published and unpublished studies were considered for inclusion.
Exclusion criteria
Articles with low quality, unclear methodologies and articles that didn’t indicate the outcome of interest were excluded (online supplemental additional file 2). Excluding the studies whose full-text papers were not available after at least two personal email contacts with the corresponding authors was an exclusion criterion but all full texts were available.
Supplemental material
Assessment of outcome variables
There were two outcomes in this study: the first outcome was the magnitude of confirmed breast cancer disease/morbidity among patients with cancer diagnosis. This outcome can occur in any population group. Therefore, the population for this outcome was a human population of any age evaluated for cancer disease.
Breast cancer
The diagnosis of breast cancer was used when diagnosis was confirmed by pathological tests in addition to the history and physical examination.16 Hence, the data were sought if the diagnosis of breast cancer was confirmed by pathological tests. The total number of people who had breast cancer was divided by the total number of people participating in the study and multiplied by 100 which was used to determine the proportion of breast cancer morbidity.
The second outcome/variables of this review were the determinants of breast cancer. Modifiable and non-modifiable factors were searched from the literature to pool their value together. For the variables whose categorisation didn’t overlap (eg, age category), the category repeatedly reported in the studies or the established categorisation was assumed to get the privilege.
Early age at menarche
Early age at menarche is the starting of menstruation early and mostly before the age of 12 years.17 18
Late menopause
Late menopause is delayed age at menopause which is after the age of 55 years in most cases.17
Benign breast disease and breast injury
These are those breast diseases such as atypical ductal hyperplasia or lobular carcinoma.17
Menopause status
Menopause status is categorised as postmenopausal if the woman has already stopped menstruation (either absence of menstruation for at least 1 year (any age) or due to bilateral oophorectomy or oestrogen deprivation therapy) and premenopausal otherwise.16 19 20
Body mass index
Body mass index (BMI) is an index which is determined based on the measurement of weight and height and is classified as high if the value is ≥25 kg/m2.16
Age (<30 years, 30–49 years, >50 years), 17 20 residence (rural vs urban), occupation (unemployed vs employed), exposure to smoking dried meat, use of industry processed foods, lack of intake of milk, fruits and sea foods, high cholesterol (total cholesterol > 200 mg/dL),21 energy source;fuel source (wood/charcoal/kerosene/animal dung vs electricity), lack of physical activity, contraceptive use, family history of cancer, history of abortion, absence of breastfeeding, benign breast disease and breast injury, exposure to radiation, anaemia and thrombocytosis were also the variables for which data were sought in the literature.
Study selection and data extraction
All the articles searched from the databases were imported into EndNote V.X7, and duplicates were removed. Based on the predefined inclusion criteria, two authors (ATS and AED) independently assessed and identified papers by their titles, abstracts and full texts. The screened items were then compiled, and any disagreement was handled by inviting and discussing with the third author (DRT). Data extraction was performed using the Joanna Briggs Institute (JBI) data extraction format.22–24 The data extraction format included the primary author, publication year, study period, region, study area, study setting, study design, study population, publication status, sample size, response rate and the number of cases of breast cancer. For the second outcome, data were extracted into a two-by-two table.
Quality assessment
JBI meta-analysis of statistics assessment and review instrument quality appraisal tool was used to assess the quality of the articles.24 The JBI parameters included an appropriate sampling frame, proper sampling technique, study subject and setting description, sufficient time to exposure measurement, use of valid methods for the identified conditions, a valid measurement for variables and conditions, using appropriate statistical analysis including control of confounding. Accordingly, quality was categorised as low (total score of ≤2), moderate (total score of 3–4) or high (total score of >5) in terms of their likelihood.24 The quality of the included studies was assessed by two independent authors (ATS and DRT). The discrepancy during the quality appraisal of the studies was resolved by the agreement of the two reviewers. Finally, papers with an overall quality score of <37.5% and/or those not reporting the outcome of interest were excluded from the systematic review and meta-analysis (online supplemental additional file 2).
Data synthesis strategy
The data were extracted into Microsoft Excel. Then it was exported to STATA software, V.14, for further analysis. The SEs of the included studies were calculated using the formula 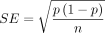 . The I2 statistics and the values of p of the Cochrane Q test were used to identify the heterogeneity problem. The values of p of the Cochrane Q test <0.1 were used to indicate the presence of heterogeneity among the studies. The Higgins I2 test statistics was used to calculate the percentage of total variance due to heterogeneity across the studies. Heterogeneity was declared for the I2 value >20%.23 As a remedial measure for the heterogeneity among the studies by the test statistic, the DerSimonian-Laird’s impact was evaluated using a random-effects model.25 Moreover, the subgroup analysis by region, study design, study setting and study population was done to identify the possible source of heterogeneity. The effect sizes were expressed as proportions and ORs along with a 95% CIs. Moreover, the 95% prediction interval was computed by using the comprehensive meta-analysis to indicate the true proportion in the comparable population.26 Forest plots were used to display the results of the meta-analysis. Publication bias was investigated graphically using a funnel plot and statistically using Egger’s weighted regression and/or Begg’s rank correlation tests and decided as significant at a value of p<0.05.27 28 A leave-one-out sensitivity meta-analysis was used to assess the robustness of the findings.
. The I2 statistics and the values of p of the Cochrane Q test were used to identify the heterogeneity problem. The values of p of the Cochrane Q test <0.1 were used to indicate the presence of heterogeneity among the studies. The Higgins I2 test statistics was used to calculate the percentage of total variance due to heterogeneity across the studies. Heterogeneity was declared for the I2 value >20%.23 As a remedial measure for the heterogeneity among the studies by the test statistic, the DerSimonian-Laird’s impact was evaluated using a random-effects model.25 Moreover, the subgroup analysis by region, study design, study setting and study population was done to identify the possible source of heterogeneity. The effect sizes were expressed as proportions and ORs along with a 95% CIs. Moreover, the 95% prediction interval was computed by using the comprehensive meta-analysis to indicate the true proportion in the comparable population.26 Forest plots were used to display the results of the meta-analysis. Publication bias was investigated graphically using a funnel plot and statistically using Egger’s weighted regression and/or Begg’s rank correlation tests and decided as significant at a value of p<0.05.27 28 A leave-one-out sensitivity meta-analysis was used to assess the robustness of the findings.
Patient and public involvement
No patient was involved in this study.
Result
Description of included studies in the systematic review and meta-analysis
About 1644 articles were identified through database searching while 17 of them were included in this systematic review and meta-analysis (figure 1). The total number of participants was 16 055.
Flow chart of the selection of studies for the systematic review and meta-analysis of breast cancer and its determinants in Ethiopia.
The studies covered the period between 2011 and 2021;16 18–21 29–40 11 were cross-sectional,21 29–38 6 were case-control,16 18–20 39 40 16 were published,16 18 19 21 29–40 1 was unpublished,20 the majority (15) were institution-based studies,16 18–21 29–33 35 36 38–40 and 2 of them were population-based studies.34 37 The majority of them (10) were conducted in Addis Ababa,16 18 19 29 32 34 35 37–40 followed by southern nations, nationalities and peoples (SNNP) (3 studies)20 31 33 and Amhara region (3 studies)21 30 36 (table 1).
Descriptive summary of 17 studies included in the meta-analysis to estimate the magnitude of breast cancer and its determinants in Ethiopia
Prevalence of breast cancer in Ethiopia
From the total of the 17 included studies, 10 articles were useed to pool the prevalence of breast cancer.16 18–21 29–40 Accordingly, the pooled proportion of breast cancer morbidity among those patients evaluated for cancer in Ethiopia was found to be 20.58% (95% CI 17.25%, 23.90%; I2=93.8%, p<0.000). The 95% prediction interval is located between 8% and 34%. This indicates that the true magnitude in 95% of all comparable populations falls in the interval between 8% and 34%26 (figure 2).
Forest plot of the pooled proportion of breast cancer among patients with cancer in Ethiopia. Note: Weights are from the ramdom-effects model.
Subgroup analysis
Since significant heterogeneity was found when pooling the magnitude of breast cancer morbidity, subgroup analysis was done to further check for the source of heterogeneity. For the subgroup analysis by region, the proportion of breast cancer was found to be 21.76%; 95% CI 17.27%, 26.2% in Addis Ababa, 21.64%; 95% CI 15.02%, 28.27% in SNNP, and 14%; 95% CI 11.10%, 16.90% in Amhara (online supplemental additional file 43). The result of subgroup analysis by study setting showed that the pooled magnitude of breast cancer morbidity was high; 22.58%; 95% CI 21.45%, 23.72% for population-based studies while 19.84%; 95% CI 15.03%, 24.65% for institution-based studies (online supplemental additional file 4). Furthermore, the subgroup analysis was done by the study population. Accordingly, the pooled magnitude of breast cancer is found to be 26.14%; 95% CI 19.87%, 32.42%, 19.05%; 95% CI 15.91%, 22.18% and 10.0%; 95% CI −8.59%, 28.59% among women with cancer, among general patients with cancer and among children who had cancer, respectively (online supplemental additional file 5).
Supplemental material
Supplemental material
Supplemental material
Publication bias
The funnel plot appeared symmetrical indicating the absence of publication bias (online supplemental additional file 6). Egger’s test (p=0.533) and Begg’s test (p=0.727) also confirmed this because they both are non-significant for a value of p>0.5.
Supplemental material
Sensitivity analysis
A leave-one-out sensitivity analysis was done to test the reliability of the findings. According to the sensitivity analyses output, the random-effects model was robust, and no single study affected the pooled proportion of breast cancer morbidity (online supplemental additional file 7).
Supplemental material
Determinants of breast cancer
In individual studies, factors like young age, age at menarche, residence, occupation, exposure to smoking dried meat, use of processed foods, lack of intake of milk, fruits, and eating sea foods, high cholesterol, fuel source (wood, charcoal, kerosene, animal dung), lack of physical activity, menopause, contraceptive use, family history of cancer, history of abortion, benign breast disease and breast injury, radiation exposure, absence of breastfeeding, high BMI, anaemia and thrombocytosis were found to be the determinants of breast cancer. From these, age, age at menarche, use of processed foods, high cholesterol, lack of physical activity, menopause status, family history of cancer, absence of breastfeeding, and BMI were reported to be statistically significant in more than one study and were pooled together. However, only seven factors showed statistical significance in the meta-analysis. Accordingly, those people who consume processed foods/drinks have 2.12 (pooled odds ratio (POR)=2.12, 95% CI 1.41, 3.17, I2=0.0%, p=0.826) times more odds of breast cancer than their counterparts (online supplemental additional file 8). This meta-analysis also revealed that the risk of breast cancer is increased by 4 (pooled odds ratio=4.08; 95% CI 2.75, 6.07, I2=0.0%, p=0.888) in the presence of high cholesterol including solid oil as compared with low cholesterol (online supplemental additional file 9). Those individuals who are physically inactive had 3.27 (pooled odds ratio=3.27; 95% CI 1.80, 5.94, I2=65.2%%, p=0.090) times more odds of breast cancer than their counterparts (online supplemental additional file 10). The pooled odds of breast cancer is 2.25 (pooled odds ratio=2.25; 95% CI 1.63, 3.10, I2=0.0%, p=0.433) times more likely in postmenopausal women than premenopausal women (figure 3). In another way, the pooled odds of breast cancer is 3.65 (pooled odds ratio=3.65; 95% CI 0.85, 15.71) times more likely for those who have a family history of cancer as compared with those without a family history of cancer (online supplemental additional file 11). Regarding BMI, when compared with those people having normal BMI, high BMI was associated with 2.27 (pooled odds ratio=2.27; 95% CI 0.85, 6.03) times increased odds of breast cancer (online supplemental additional file 12). Those women who had no history of breastfeeding have 2.76 (pooled odds ratio=2.76; 95% CI 0.90, 7.92) times more odds of breast cancer compared with their counterparts (online supplemental additional file 13).
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
The pooled OR showing the association between menopausal status and breast cancer in Ethiopia.
Discussion
In total, 24 articles were assessed for inclusion and 7 were excluded from this review. The reason for exclusion was mainly the lack of an intended outcome report and non-similarity of the study population. Here are the citations of excluded studies.41–47 Data of 17 articles were extracted: 10 for prevalence and 7 for determinants. Accordingly, the pooled proportion of breast cancer morbidity in Ethiopia is found to be 20. 58% (95% CI 17.25%, 23.90%). Although the result seems to be low when compared with the result in Iranian women (23.6%),48 it is still high when compared with the age-standardised incidence rate of breast cancer in Ethiopia (12.1 per 100 000 population).5 The observed variation could be due to a difference in the denominator. As shown in the subgroup analysis, the proportion of breast cancer morbidity varies in different situations. For example, the proportion of pooled breast cancer morbidity is higher (26.14%) in women diagnosed with cancer, which is even higher than the one in Iranian women48 and 19.05% among general patients with cancer. This shows that breast cancer varies depending on the study population. In another way, this finding is low as compared with the breast cancer cases (25%) among women newly diagnosed with cancers in the GLOBOCAN 2012 Study and GLOBOCAN 2018 Study49 50 and the study in the USA (29%).51 The difference in socioeconomic and demographic conditions might be the possible reason for this variation. Developed countries have improved cancer detection, registration and reporting as compared with Ethiopia, which could show a difference in breast cancer proportions between countries. Although the prevalence of breast cancer morbidity seems low when compared with developed settings, this result is still high in comparison with the previous estimation. This alerts us to the fact that breast cancer deserves attention, especially in women. The subgroup analysis in this study also indicates a high prevalence of breast cancer disease among women who suffers from different forms of cancer.
In this systematic review and meta-analysis, factors such as the use of processed foods/drinks, high cholesterol, lack of physical activity, postmenopausal status, family history of cancer, absence of breastfeeding and high BMI including obesity were reported as risk factors for breast cancer. Accordingly, a family history of cancer including breast cancer was reported as a risk factor for breast cancer (pooled odds ratio=3.65; 95% CI 0.85, 15.71). This finding is consistent with previous studies conducted in Ethiopia,14 Iran,52 53 the UK,54 China55 and Malaysia.56 This might be due to the presence of some inherited defect that could facilitate the development of the disease. Given that biological exposure is non-modifiable, screening and follow-up of the breast condition would help to get timely treatment that can halt the bad consequences of the disease.14
This study also revealed non-breastfeeding as a risk factor for breast cancer which is in line with the findings of a previous systematic review,14 57 studies done in China,55 Iran52 and the USA,58 59 where studies conducted stated that breastfeeding minimises the risk of breast cancer. The possible reason could be because of the hormonal effect of breastfeeding for the protection or reduction of breast cancer. Both the current study and previous studies revealed the protective effect of breastfeeding for breast cancer. The possible mechanism for the observed protective probability of breast cancer in this study might be attributed to the differentiation induced to the breast lobe by lactation that might transform cancer-prone stem cell 1 to refractive stem cell 2.60 There might also be less exposure of breast tissues to hormones as breastfeeding inhibits ovulation and the hormones from the ovulation cycles.61 The result is a good indicator for the promotion of breastfeeding which has dual benefits for both the mother and the child.
This current finding also showed that high BMI is a risk factor for breast cancer in which people with high BMI were about 2.27 times more likely to develop breast cancer than their counterparts. This finding is similar to the previous study,56 59 62–65 and studies in Iran,52 53 but contrasts with the finding of the study conducted in northern California.66 High BMI including obesity is found to be a risk factor for breast cancer in postmenopausal women.67–69 Increased body fat might increase the level of circulating oestrogens and decrease the levels of sex hormone-binding globulin.70 Besides, the inflammation that accompanies obesity might also contribute to breast cancer development.71
In this study, lack of physical activity was found to be a risk factor for breast cancer and this has also been reported in previous studies.52 72 73 The possible explanation for the association between breast cancer and lack of physical activity might be that physical inactivity could increase the probability of fat accumulation in the body as some studies52 53 found that obesity is a risk factor for breast cancer. Therefore, adherence to regular physical exercise and healthy food would help to control weight and reduce body fat given that both overweight and physical inactivity are the two modifiable and related risk factors for breast cancer.
This study revealed that the use of processed food and/or drinks was a risk factor for breast cancer. This is consistent with the findings of the individual studies conducted in Latin America,74 Iran75 and in other reviews.63 76 According to this study, consumption of packed food/drinks was found to be a risk factor for breast cancer. This result is in line with the study findings of other countries which imply that a decrease in the intake of packed or ultraprocessed food or drink should be encouraged to reduce the incidence of breast cancer among women.63 74–76 The possible reason for this association might be due to the presence of different additives to processed foods during the processes that could initiate cancer development.77 Other reasons might be that packed foods are rich in energy/added sugar, saturated and transfatty acids, and salt and have low fibre content and vitamins, that would increase the risk of breast cancer.78
In this review, a positive association between high cholesterol level and breast cancer was found. However, studies are contradictory in this regard. Some studies found high cholesterol as a risk factor79 while some found it as a protective factor.80 81 Those studies that found the protective effect of high cholesterol explained it as ‘statin-the cholesterol-lowering medication might reduce the breast cancer risk too’.82 83 In this study, the total cholesterol including the use of hard oil was used as high cholesterol and was found to be associated with increased breast cancer risk. The possible reason for the positive association between high cholesterol and breast cancer is that cholesterol is the precursor for oestrogen which is the cause of breast cancer.84 85 Women with high body fat might have an increased risk of breast cancer though their BMI is normal.
Moreover, the current study revealed that postmenopausal status is one of the risk factors for breast cancer which is consistent with the previous studies14 86 which indicated that breast cancer risk is higher in postmenopausal than premenopausal women. However, this result seems to contradict the finding in another meta-analysis in which premenopausal women had about 43% higher risk of breast cancer than postmenopausal women of the same age. In another way, that study added that postmenopausal women with high body fat had an increased risk of breast cancer than premenopausal women.69 Hence, the association between the increased possibility of breast cancer and postmenopausal status in this study might be justified as those postmenopausal women could have high body fat as well. Another possible explanation is that postmenopausal women in this study might have reached menopause at later age commonly after 50 years as late menopause is found to be a risk factor in another study,55 because extended menstruation could lead to increased exposure of the breast tissue to hormones like oestrogen.63 Besides, the use of postmenopausal hormone replacement therapy couldn’t be ruled out from the possible reasons as this might increase the risk of breast cancer in postmenopausal women.57 This implies that the hormonal change in premenopausal and postmenopausal women contributes to a risk or solution for breast cancer. Although there are differences in explanatory models regarding the postmenopausal stage, it is imperative to care for oneself because age extremes are mostly known to have a high risk for disease including chronic conditions like breast cancer.17
This study has its own implications for research, practice and policy. The practical implication is that a programme on non-communicable diseases like breast cancer in the country should be strengthened to combat the problem given the prevalence in this study is high. On top of that, a protective effect of breastfeeding found in this study implies that programmes that address breastfeeding promotion should incorporate the protective role of breastfeeding in their promotion activities. This research also alerts future research to investigate factors like residence, occupation, smoke-dried meat consumption, unclean energy sources, and the protective effects of foods like milk, seafood, and fruits including their risk and protective mechanisms. The issue of postmenopausal status is still non-conclusive in the literature. It deserves further analysis to put under its appropriate classification (risk or protective factor for breast cancer). Moreover, researchers and policy makers should work together on how to intervene and prevent the consumption of globalised and commercially processed foods as they are contributing to the breast cancer burden. The infection prevention policy of the country should be revisited to incorporate the prevention of non-communicable diseases. Although the disease is partly due to non-modifiable risk factors, the presence of modifiable factors calls for all concerned bodies to focus on the disease to prevent the disease, diagnose and treat timely, and minimise the risk of death and economic impact. This may include the initiation and strengthening of breast cancer screening in the country.
The strengths of this study are that it is the first systematic review and meta-analysis on breast cancer in Ethiopia which pooled the prevalence of breast cancer. Next, it shares the strengths of systematic review and meta-analysis as the evidence generated from this systematic review and meta-analysis might be more representative of the country’s situation than pocket studies. Third, the study estimated the prediction interval for the result obtained which is uncommon in many previous meta-analyses. However, the review was not free of limitations. The first limitation could be the narrow scope of the review in which a single country is covered. Nonetheless, the studied country can use the results to consider their policy decisions. The other drawback was that some of the regions had no primary studies regarding breast cancer and were not included in this review. The majority of the studies were done in Addis Ababa city which is the country’s capital. Another drawback is that the evidence pooled together was merely from observational studies (cross-sectional and case-control studies).
Conclusion
In Ethiopia, out of five patients evaluated for cancer disease, one received a diagnosis of breast cancer. Additionally, more than a quarter of cancer disease in women is breast cancer, according to this study. In another way, the use of processed foods, high BMI, high cholesterol, physical inactivity, postmenopausal status, family history of cancer and lack of breastfeeding were the facilitators of breast cancer development.
Postmenopausal women, in particular late menopause women, should stick to the lifestyle modifications that help to control body fat. It would be better if the people of Ethiopia use food sources such as fruits and vegetables, homegrown varieties of crops and the like, rather than seeking to adopt the westernised food culture (processed foods). It is highly recommended to practice regular physical exercise to regulate body weight, and body fat and then to protect against the risk of breast cancer. Appropriate breastfeeding should be practised for at least 2 years after delivery as this contributes to the minimisation of breast cancer risk. Regular breast examination should be practised to detect and control the problem timely.
Data availability statement
Data are available upon reasonable request. The data extracted from included studies and analysed in this review are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @adisu Tafari Shama
Contributors ATS conceptualised the study, designed the methods, wrote the protocol, searched, screened and critically evaluated the studies, extracted the data, analysed the data, and wrote the manuscript. DRT was involved in the critical appraisal of the studies and data extraction, and wrote the first draft of the result. AED searched, screened and critically appraised the studies, extracted the data, and interpreted the result. ML searched and screened the studies, drafted the methods and wrote the introduction for the study. MCC and ETG extracted the data and prepared the manuscript. JWF, BRF and BB designed the methods, searched the studies, and extracted and analysed the data. ATS is the guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.





