Article Text
Abstract
Objectives Medical adhesives provide securement of medical devices, facilitate skin protection and allow non-invasive monitoring. Application and removal of medical adhesives can result in pain, dermatitis, trauma or other skin lesions. Understanding patients’ experiences when subjected to medical adhesives will contribute to the improvement of clinical routines and the development and improvement of new adhesive technologies. A qualitative systematic review was conducted to identify patients’ experiences with the application of medical adhesives to the skin.
Design Qualitative systematic review.
Data sources CINAHL, EMBASE, MEDLINE and PsycINFO were systematically searched for records published between January 2012 and March 2024. Reference lists of systematic reviews and included articles were reviewed.
Eligibility criteria Studies published in Danish, Dutch, English, German, Norwegian and Swedish that collected qualitative data on the experience of patients with the application of medical adhesives to the skin were considered. There were no restrictions regarding age, gender or setting.
Data extraction and synthesis Study selection, data extraction and quality appraisal were independently conducted by two reviewers. The methodological quality of the studies under consideration was assessed using the Joanna Briggs Institute Critical Appraisal Tool for Qualitative Research. The extracted data were synthesised using meta-aggregation.
Results Nine studies describing patients’ experiences were included. The included studies only reflected experiences with wound dressings. Meta-aggregation of the extracted findings resulted in seven categories that were further synthesised into two synthesised findings: ‘strategies to alleviate pain during dressing changes’ and ‘dressing construction and characteristics’. The synthesised findings illustrate that patients experience pain during dressing change and removal and employ various strategies to alleviate this pain.
Conclusions Patients experience pain and discomfort when dressings are changed or removed. Future research should focus on enhancing both routines and technologies, with a particular emphasis on advancing skin-friendly adhesives to reduce unwanted side effects.
PROSPERO registration number CRD42023457711.
- Systematic Review
- PAIN MANAGEMENT
- Patients
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Using meta-aggregation as a method for qualitative data synthesis ensures a comprehensive, systematic approach to summarising patients’ experiences.
Though only four databases—MEDLINE, CINAHL, EMBASE and PsycINFO—were systematically searched, potentially limiting the comprehensiveness of this review, these are the largest and most relevant databases to the field.
This systematic review considered studies published in Danish, Dutch, English, German, Norwegian and Swedish, enhancing the comprehensiveness of this review and reducing the risk of language bias.
The study selection, data extraction and quality appraisal were performed in duplicate, which strengthens the reliability and minimises potential bias.
Introduction
Medical adhesives are defined as adhesives used in medical devices to establish and maintain contact with the body over a period of time (usually by application to the skin). They are a component of a variety of products, including bandages and dressings for wound care, ostomy supplies and patches, adhesive film or tape to secure various catheters, tubes and electronic devices (eg, adhesives used for securing ECG and EEG electrodes to the skin).1 2 Medical adhesives are frequently used in an array of healthcare settings in all patient groups. From premature babies, who often require medical adhesives to secure nasogastric and ventilation tubes, to patients with an ostomy who frequently have to reapply the adhesive stoma products to their skin. In an acute care facility in the USA, a median of 3.00–6.25 adhesive products was used on the skin per patient per day.3
Patients may experience pain when changing the medical adhesive.4 5 The patient’s perception of pain is influenced by several factors such as mental and physical health conditions, previous negative experiences and types of medical adhesive used.6 Therefore, it has been recommended to perform a pain assessment at every dressing change.7 Pain and discomfort can cause chronic stress, which might result in impaired wound healing.8 9 Especially in children, pain can lead to emotional trauma and even post-traumatic stress,10 11 which potentially results in avoidance of trauma reminders and negative moods or cognitions.12 Pain is defined as ‘an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage’.13 Activation of nociceptors in the epidermis sends signals about potential or actual tissue damage, which causes the experience of pain.14 This starts an autonomic stress response, which includes heart rate elevation and metabolic changes. Stress exacerbates the pain experience.14
Skin damage can cause pain and discomfort in patients.9 15–17 Application and removal of medical adhesives to the skin can lead to skin stripping, contact dermatitis, or allergic reactions that may manifest as inflammation associated with itching or pain. Adhesive-related skin injury can lead to infection, delayed wound healing and an increased risk of scarring.2 Medical adhesive-related skin injury (MARSI) occurs when the adhesive material’s adhesion to the skin is stronger than the adhesion between the skin’s cells on removal. This leads to the separation of epidermal layers or the complete detachment of the epidermis from the dermis, observed as erythema, cuts and blisters.7 Medical adhesive-related skin injuries can occur in any patient, but elderly patients and newborns are particularly susceptible.18–20
Despite the frequent use, medical adhesive-related injuries are rarely reported7. Previous studies have shown that nurses did not take action to prevent pain and skin tearing when carrying out dressing change.17 Understanding the patient’s experience with medical adhesives is crucial to determine the focus of further research, to establish policies and to raise awareness among healthcare professionals with the aim of minimising adverse effects and enhancing patient outcomes during the use of medical adhesives.
Therefore, this systematic review aimed to answer the following research question: ‘What are patients’ experiences with the application of medical adhesives to the skin?’
Methods
This systematic review is reported according to the Enhancing Transparency in Reporting the Synthesis of Qualitative Research statement criteria.21 Meta-aggregation was used to synthesise the results based on the guideline from the Joanna Briggs Institute (JBI).22 This review is registered with the PROSPERO International Prospective Register of Systematic Reviews. The protocol of this review has been published previously.23
Search strategy and information sources
A two-step strategy was used to identify relevant studies. First, a systematic search in four electronic databases was conducted: CINAHL (accessed through the EBSCO interface), EMBASE (accessed through Elsevier), MEDLINE (accessed through the Ovid interface) and PsycINFO (accessed through the EBSCO interface). For the initial searches in MEDLINE, the concepts ‘experience’ (keywords include ‘pain’, ‘dermatitis’, ‘itching’, ‘pruritus’ and ‘discomfort’) and ‘removal of dressings’ (keywords include ‘adhesive’, ‘bandage’, ‘dressing’, ‘adverse event’, ‘device deficiency’, ‘removal’, ‘change’ and ‘application’) were used. The initial search strategy was customised for each electronic database (see online supplemental file 1). Second, the reference lists of relevant systematic reviews and included articles in this review were screened to identify additional studies that were not retrieved through the first strategy.
Supplemental material
Eligibility criteria
Population and context
This review focused on patients who currently or in the past had medical adhesives applied to their skin. There were no restrictions regarding sex or age.
Phenomena of interest and study design
Studies were included in the review if they collected qualitative data on the experience of patients with the application of medical adhesives to the skin. Both qualitative studies and qualitative data from mixed method studies were considered.
Setting, language and time frame
There were no restrictions regarding settings. Articles published in Danish, Dutch, English, German, Norwegian and Swedish were considered. Due to continuous technological advances in the field of medical adhesives,24–26 this review tried to focus on medical adhesives that are currently still being used in clinical practice by restricting the search period. Therefore, the initial search was conducted to identify records with a publication date between January 2012 and November 2022. The search was repeated in March 2024 to identify any additional studies.
Study selection, data collection and management
All databases underwent individual searches, and the retrieved records were then exported into Covidence software for systematic reviews (Veritas Health Innovation, Melbourne, Australia). Following this, duplicates were identified and subsequently eliminated. The screening of records was conducted independently by two reviewers (HH, TD). In case of disagreement, discussions were held until consensus was reached. If there was no consensus, a third member of the review team was consulted (ME or DB). First, the titles and abstracts of the records were screened against the inclusion criteria. In a second round, the full text of the selected articles was screened.
Assessment of methodological quality
The methodological quality of the studies under consideration was assessed independently by two reviewers (HH, TD). The JBI Critical Appraisal Tool for Qualitative Research was used.27 In cases of disagreement, discussions were held among the reviewers to reach consensus about the methodological quality. If necessary, a third reviewer was involved to resolve remaining disagreements (DB).
Data extraction
From the included studies, (a) bibliographic information (lead author, year, title, journal, full citation) (b) study design and sample size, (c) patient demographics, setting and geographical context, (d) description of how the research findings are addressed in the article, (e) method of data collection, (f) method of data analysis, (g) context (product names/brands or type of material of medical adhesives investigated), (h) phenomenon of interest (experience of patients with the application of medical adhesives to the skin) and (i) findings and illustrations were extracted. Definitions of findings and illustrations in meta-aggregation are provided in table 1.
Key concepts and terminology in meta-aggregation.
Data extraction was independently conducted by two reviewers (HH and TD), with any ambiguities addressed through discussion within the research team. Final data extraction was accomplished through reviewer discussions, ensuring consensus was reached. Another member of the research team (ME, DB) performed quality control of the extracted data on 20% of the included articles.
Data synthesis
Meta-aggregation was used to summarise the evidence. A level of plausibility was allocated to each extracted finding: unequivocal, equivocal and unsupported. Unsupported findings do not appear in the data synthesis.22 27
Meta-aggregation was completed according to the following steps: (a) each article was read repeatedly to extract all findings from the results and discussion section of the included studies, accompanied by an illustration; next, a level of plausibility was allocated to the extracted finding, (b) findings were summarised into categories based on similarity of concepts and (c) synthesised findings were derived from categories.22 27 Category descriptions and synthesised findings were created by a consensus process among three members of the review team (HH, TD and DB), after repeated reading of the extracted findings.
Patient and public involvement
No patients were involved in the design or conduct of this systematic review.
Results
Screening and search outcome
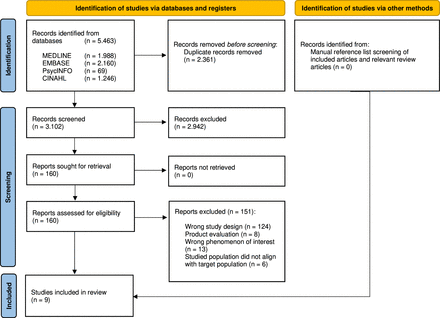
The literature search identified 5463 records. No additional records were identified through manual search. After removing duplicates, two reviewers (HH and TD) independently screened the title and abstract of 3102 articles using the software tool Covidence. The eligibility of 160 articles was assessed by screening the full texts. After full- text screening, 151 studies were excluded. In total, nine studies were included. The search and selection process is summarised in figure 1.28
PRISMA flowchart. PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols.
Description of included studies
The included studies were published between 2013 and 2023. Five studies were conducted in the United Kingdom,29–33 and one each in Turkey,34 Brazil,35 Ireland36 and China.37 Four studies adopted a phenomenological approach.30 34 35 37 Seven studies used semistructured interviews, in-depth interviews or focus groups.
Various methods for data analysis were employed across these studies. Data collection was conducted either directly from patients or through proxies such as parents, healthcare providers or informal caregivers. Sample sizes across the studies varied, ranging from 7 to 150 participants. All medical adhesives used in the included studies were wound dressings. Table 2 provides a detailed overview of the study characteristics.
Characteristics of Included Studies
Assessment of methodological quality
The quality appraisal of the nine studies showed varying quality levels. All studies used suitable methodologies, but none addressed the researchers’ cultural or theoretical background, and only one noted the potential influence of researchers on the outcomes.36 To ensure a comprehensive synthesis of the existing evidence, articles were not excluded based on low quality. Online supplemental file 2 provides a detailed overview of the assessment of methodological quality.
Supplemental material
Findings
Patients and healthcare providers reported that patients experienced pain during dressing removal and dressing changes.30 32–34 36 37 From the 9 included studies, 43 findings were extracted after repeated reading of the text. 24 of the 43 extracted findings were supported by an illustration and were, therefore, allocated unequivocal or equivocal as level of plausibility. The supported findings were then aggregated into seven categories, based on similarity in meaning.27 These categories were clustered further into two synthesised findings based on similarity of concepts: ‘strategies to alleviate pain during dressing changes’ and ‘dressing construction and characteristics’. Table 3 provides an overview of the meta-aggregation of the extracted supported findings.
Overview of meta-aggregation of the extracted findings
The category ‘emotional response to pain caused by dressing changes’ could not be clustered into any synthesised finding, since a synthesised finding has to consist of at least two categories.22 27 Current or previous experiences of pain during dressing change can trigger an emotional response in patients. Healthcare providers described non-compliance with leg ulcer treatment in patients due to anxiety and anticipated pain based on previous painful experiences. If you tell them we need to increase their visits they don’t like it because obviously they know they’re going to get pain … it kind of puts them off and then they become non-compliant.30 Patients reported that distraction by use of virtual reality (VR) gave them a sense of control over the situation, which resulted in a decrease in pain during dressing change. Something as trivial as a video was actually quite empowering for me because I could take myself away.32
Synthesised findings
Strategies to alleviate pain during dressing changes
The synthesised finding strategies to alleviate pain during dressing changes emerged from four categories: (a) ‘analgesia is a strategy to alleviate pain during dressing changes’, (b) ‘VR is a strategy to alleviate pain during dressing changes’, c) ‘strategies to alleviate pain and suffering in children caused by dressing changes’ and (d) ‘procedures to remove dressings’ (table 3).
a) Analgesia is a strategy to alleviate pain during dressing changes
Analgesia and anaesthesia were described as strategies to alleviate pain during dressing changes.30 34 36 37 Patients reported that being provided anaesthesia before the dressing changes reduced the experienced pain. On the first changing, they made me sleepy (with narcotics) and I didn’t feel anything then the wraps were taken off the skin. They didn’t anaesthetize me the second time, and it was much worse.34 Healthcare providers similarly recommended patients to take additional analgesics prior to dressing change appointments in order to reduce pain during dressing,30 36 37 even though they sometimes triggered side effects.36 Some patients gave recommendations about research on dressings or pain management to their care network. Olivia suggested focusing research on pain relieving dressings rather than drugs. Some also indicated the importance of timely referral to a pain manager.36
b) VR is a strategy to alleviate pain during dressing changes
Additionally, utilising VR was described as a strategy to alleviate pain during dressing changes. The use of VR distracted patients from focusing on the wound care and accompanying pain during dressing change. Before you were thinking, it hurts, because watching them do it makes it worse.32
c) Strategies to alleviate pain and suffering in children caused by dressing changes
Parents and healthcare providers reported pain and suffering in neonates and children during dressing change.35 37 The day I most saw her crying in pain was when she removed the tape.35 Even though pain during dressing change is a known problem, healthcare providers reported a gap between the current situation and their expectations regarding strategies to alleviate pain during dressing change in children. Patients received too little or even no pain relief.35 37 Analgesics available for children are quite few, children with burns cry all the time during the dressing, and we need available drugs or methods to relieve their pain.37
d) Procedures to remove dressings
Specific procedures for removal of dressings were described.31 34 Unver et al 34 reported that swift removal of adhesives and the resulting skin trauma were the main causes of pain during dressing changes. Patients soaked the adhesive dressings in the shower to aid dressing removal and reduce removal pain. I just completely soaked it (adhesive dressing) in the shower then my husband just took it off for me. But it was, it was really easy. Much easier than I thought.31 Patients experienced procedural pain and indicated that activities of the daily living influenced pain levels. Maybe sometimes with dressing changes, the worst pain I had was with the VAC dressing (Negative Pressure Wound Therapy).36
Dressing construction and characteristics
The two categories (a) ‘characteristics of an atraumatic dressing’ and (b) ‘adverse reactions to the dressing’ have been synthesised on the basis that they both describe the constitution of the dressings used in the studies. This synthesised finding demonstrates that dressings should be designed in a way that facilitates easy removal and minimises discomfort during wear.
a) Characteristics of an atraumatic dressing
Atraumatic application and removal were described as a characteristic of an atraumatic dressing. Those dressings helped my mum’s legs in that they didn’t hurt here when the nurse took them off.29 Additionally, skin protection of the peri-wound skin, good adherence and comfort during wear of the adhesive dressing were highlighted as features of atraumatic dressings. Very important not to have them stuck on the area that has just been healed, and it is very difficult to take it off without hurting the wound again, and I think that is terribly important.29 30
b) Adverse reactions to the dressing
To minimise discomfort during dressing wear, potential adverse reactions to dressings must be considered when choosing an adhesive dressing. Frequent dressing changes due to leakages caused by highly exudating wounds, were reported as very painful. It is excruciating when the dressings keep coming on and off and she is in unbearable pain (reported by carer).33 Itching and allergic reactions to the adhesives used were also described as uncomfortable adverse reactions to an adhesive dressing. I’ve now got really itchy where the plaster goes. Which is uncomfortable.31
Discussion
This systematic review aimed to synthesise patients’ experiences with the application of medical adhesives to the skin. This systematic literature search only retrieved studies that included findings on wound dressings. No records reporting patients’ experiences with other types of medical adhesives such as ECG electrodes, intravenous catheter patches, securement for medical devices, ostomy supplies et cetera were identified. All included studies in this review reported experiences with the changing and removal of dressings. No findings described patient experiences with the application and wear of adhesive dressing.
The results imply that patients experience pain and discomfort during dressing change and removal.30 32–34 37 Awareness among healthcare providers is important since a single painful experience can change nociceptive pathways and induce sensitisation. This is a process that involves a reduction in the threshold of activation and an increased response rate to damaging stimulation.38 39 Pain is a personal experience, influenced by biological, psychological and social factors to varying degrees.13 A clinical tool predicting severe pain (Numeric Rating Scale ≥8) during wound dressing changes using clinically available wound and patient factors was developed.40 41 Expected pain intensity (p<0.001; OR=1.50), resting pain intensity (p<0.001; OR=1.19) and type of dressing (p<0.05; OR 1.19 to 3.62) are significant predictors for experiencing high-intensity pain during wound care procedures (overfitting-corrected AUC=0.826). Sex, age, ethnicity chronic pain, opioid tolerance, anxiety, depression and pain catastrophising were not significant predictors.41 Pain catastrophising is measured by using ‘the pain catastrophising scale’ and the term is frequently used since the factors included in the measurements are a comprehensive predictor of pain. However, this term is controversial since people with chronic pain have reacted negatively towards it as the term diminishes the importance of the medical reason behind their pain and focuses too much on psychological factors, which in the end can lead to insufficient care.42 Through the use of neurological imaging, cortical and subcortical pathways have been identified that are activated when the patient expects pain. This is called anticipatory pain.43 Patients experiencing anxiety in relation to anticipatory pain can develop a reduced pain tolerance and lead to an increased self-reported pain intensity, resulting in more painful future procedures.40 43 44
Along with describing experiences, patients and proxies describe the need for strategies to alleviate the pain and discomfort experienced during the application of dressings to the skin.30–32 34 35 37 Both pharmacological and non-pharmacological interventions to alleviate dressing-related pain were described. Healthcare professionals describe the lack of an appropriate analgesic regimen for neonates needing their burn wounds dressed.37 Many infants get too little or no pain relieving interventions despite the existence of validated pain assessment tools and recommended actions for pain management when conducting medical procedures. The recommendation for neonates is both pharmacological measures, such as acetaminophen, opioids and local topical agents, and non-pharmacological measures, such as breast feeding, skin-to-skin contact and sucrose solution together with non-nutritive sucking.45 In addition, distraction by VR was described as a non-pharmacological intervention to reduce dressing change-related pain.32 Immersive VR has been demonstrated to alleviate pain across various medical procedures, including dressing changes in patients with hand injuries.46 For patients to take prescribed analgesics before dressing changes and for nurses to recommend patients to take analgesics before dressing changes was also part of the synthesised finding.30 36 Recommended pharmacological strategies for treating pain or breakout pain when changing dressings include increasing the dose of the analgesic already prescribed, adding another fast-acting pain medication or reducing the time in between doses.47
Health professionals should improve their communication with patients about the risks related to adhesive wound dressing use. They should try to minimise pain during dressing removal and the occurrence of MARSI.7 It is important for health professionals to understand the unique characteristics of an adhesive wound dressing for informed decision-making regarding the selection of the dressing.48 Dressing characteristics for atraumatic dressing removal were described in a few studies.29–33 Patients with atraumatic dressings using a silicone contact layer applied to their skin report significantly lower pain scores (p<0.01) when compared with traditional adhesives (ie, adhesive foams, hydrocolloids and other dressings).49 It is also important for health professionals to have knowledge about the skin as well as knowledge about application and removal techniques for adhesive wound dressings and medical adhesives in general to prevent unnecessary damage to the patient.48 The barrier function of the skin can be damaged as a result of single or repeated application of adhesives, despite a reduction in adhesive strength during prolonged dressing wear.50
Methodological considerations
This review used meta-aggregation to synthesise the findings. No member of the research team had previous experience with this data synthesis method. Therefore, meta-aggregation was performed independently by two members of the research team (HH and TD). Extracted findings were synthesised to a higher level of abstraction until consensus was reached. When necessary, a third member of the research team (DB) was consulted.
The methodological quality of the included studies was assessed, but no studies were excluded for low quality. However, all studies lacked reflexivity regarding researchers’ cultural and theoretical backgrounds, with only one study addressing the potential influence of the researchers on the outcomes. Methodological guidelines for qualitative research recommend that researchers reflect on their own position, biases and assumptions in their writings before and during the research process to minimise bias.51 The lack of a statement on reflexivity in the primary studies may indicate bias, as readers of these articles are not informed about the authors’ perspectives and prejudices regarding the concept of pain before they started the analysis process.
Strengths and limitations
The systematic review only included studies containing qualitative data to explore patients’ experiences with the application of medical adhesives to the skin, which resulted in only nine eligible studies. Employing quantitative studies in addition to qualitative articles might have provided interesting insights into pain and discomfort scores of patients while adhesive dressings are being removed. Conducting a mixed-method review has several limitations, including difficulties in comparing results from these different paradigms and extending the time required to complete the review.52
For this review, only four databases were systematically searched. MEDLINE, CINAHL and EMBASE are among the largest and most relevant databases in the field of nursing science. PsycINFO primarily covers psychology, behavioural science and mental health. These databases were selected to ensure comprehensive coverage of primary studies containing qualitative data on patients’ experiences with the application of medical adhesives to the skin. Their scope makes them the optimal choice for capturing the most relevant studies for the data synthesis.
Only studies published between January 2012 and March 2024 were considered. The initial search for this systematic review was conducted in November 2022, focusing on articles published between January 2012 and November 2022. The search was updated in March 2024 to capture any new publications on the topic of this review. In light of the ongoing advancements in medical adhesives and technological innovations,24–26 this study aimed to focus on adhesives currently used in clinical practice. Additionally, during the last 10 years, pain research has advanced significantly.13 Limiting the timeframe from January 2012 to March 2024 enabled incorporation of the latest knowledge and developments in the field.
The study characteristics of the included studies, such as age, setting and country, were heterogeneous. Since only a limited amount of findings could be extracted, it was not possible to identify potential cultural differences in the reported findings.
Studies that were published in languages other than Danish, Dutch, English, German, Norwegian or Swedish were not screened through the search strategy. This may have led to the exclusion of relevant articles published in another language.
Four of the included studies30 32 34 37 did not specify the used dressing type or brand. No additional information on dressing type or brand was retrieved by contacting the authors. As a result, not all of the published information could be synthesised fully.
This systematic review describes patients’ experiences with the application of dressings on various wound types: burn wounds,32 37 chronic leg ulcers,30 surgical wounds31 34 and epidermolysis bullosa.33 Pain can also be caused by tissue damage.53 Reported experiences of pain and discomfort with the application of medical adhesives to the skin might consequently be obscured by wound pain.36 41
This study did not involve patients or the public in its conceptualisation, design or conduct. This qualitative systematic review is part of a larger research project, the TAPE research project, which consists of four phases. In the subsequent phases of this project, patients will be involved in refining the research objectives to ensure the concerns of patients who use medical adhesives are addressed.
Implications for research and clinical practice
Future research should focus on exploring routines to reduce unwanted side effects with medical adhesive use in clinical practice. This will guide improvement of adhesive technologies, the establishment of policies and raise awareness among healthcare professionals regarding the pain and discomfort related to medical adhesives application to the skin.
Pain and discomfort related to the application, presence and removal of medical adhesives are often overlooked. A lack of established policies and training exacerbates the issue. Pharmacological interventions designed to alleviate pain and discomfort related to the application, use and removal of medical adhesives often result in unwanted side effects. Non-pharmacological interventions offer alternatives but costs of necessary equipment, such as VR materials, may result in a limited availability. Establishing policies and raising awareness among healthcare professionals is needed.5 17 This can be done through an educational effort as well as raising awareness on a higher level in the healthcare system, for example, questioning the materials being bought for hospital wide use. When cost is the deciding factor, it is important to evaluate whether different brands offer comparable adhesion and skin protection.
Future research should focus on enhancing both routines and technologies, with a particular emphasis on advancing skin-friendly adhesives to reduce unwanted side effects. Interviewing patients about their experiences and doing a narrative description of specific aspects of the dressing change process could be of value. Since medical adhesives are frequently used in all patient groups and the findings of this study indicate that patients experience pain when dressings are being removed, future qualitative research should explore patient experiences with other types of medical adhesives (ECG electrodes, intravenous patches, et cetera).
Future dressing development should focus on material science, cell biology an intelligent technology to develop multipurpose dressings that can further improve wound management.54 In some cases, there will be a need for medical adhesives that adhere more strongly to the skin to prevent dislocation of life-saving medical devices such as endotracheal tubes and intravenous catheters in an intensive care setting.
Conclusion
The meta-aggregation performed in this study implies that patients do experience pain and discomfort when wound dressings are changed or removed. The synthesised findings of this review ‘strategies to alleviate pain during dressing changes’ and ‘dressing construction and characteristics’ can serve as a guide to improve clinical routines for adhesive dressing use, avoid pain and discomfort while changing adhesive dressings4 5 and prevent emotional trauma and post-traumatic stress in children.10 11
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
Mölnlycke Health Care AB provided financial support to conduct this systematic review. We would also like to thank Samal Al Gilani, RN, PhD, for participating in the planning of the project and in some additional screening.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors HH and TD contributed equally to this paper. All authors contributed to the conception of the research question and the writing of the protocol. HH, DB, SJ, JK, L-MK and ME contributed to the development of search strategies, eligibility criteria and methodology for data synthesis. All authors contributed to the draft protocol and approved the final version of the protocol. HH, TD and ME worked in duplicate to review the titles and abstracts of all materials obtained using the search strategy to exclude articles that do not meet the eligibility criteria. HH and TD evaluated potentially eligible studies through full-text screening and excluded non-eligible studies, documenting the reason for exclusion. HH and TD independently extracted data from the included studies. ME and DB checked the quality on 20% of the extracted data of the included articles. HH and TD synthesised the data and drafted the manuscript. All authors read, provided feedback and approved the final manuscript. ME is responsible for the overall content as guarantor.
Funding This systematic review is supported by Mölnlycke Health Care AB, grant number 68003673. Mölnlycke Health Care AB was not involved in any other aspect than the funding of this systematic review. The funder had no input on the interpretation or publication of the study results.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer-reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.