Article Text
Abstract
Introduction Insomnia is the most common sleep disorder, and it adversely impacts daily living and increases the risk of chronic and acute health problems. Of the few individuals who seek treatment for insomnia, most pursue help in primary care settings. The management of insomnia most commonly focuses on the prescription of hypnotics and sleep hygiene recommendations, although these are not the most effective treatments. Conversely, cognitive–behavioural therapy for insomnia (CBT-i), which is considered to be the first-line treatment for persistent insomnia, is seldom prescribed by primary care physicians (PCPs) or primary care nurses (PCNs). The hesitancy of these professionals to provide CBT-i is mainly attributed to their heavy workloads and the difficulties in acquiring the skills needed to administer this intervention.
Methods and analysis A two-arm cluster-randomised study (in which patients are assigned to a PCP or PCN) will be conducted in primary health centres of Majorca Island (Spain). A total of 206 patients will be recruited. Healthcare professionals will be allocated to the intervention or control group in a 1:1 ratio. The intervention group will receive CBT-i and the control group will receive usual care. We will include patients with Insomnia Severity Index scores of 8 or more who also report that insomnia interferes with daily functioning or is noticeable to others. The CBT-i will consist of four individual structured sessions, three in person (20 min each) and one by telephone (10 min) that are administered at intervals of 2–3 weeks. An additional session will be provided for patients taking hypnotic medications. The primary outcome measure is the decrease in sleep latency, which will be measured with the Pittsburg Sleep Quality index at 6 months and 12 months.
Ethics and dissemination This project was approved by the Ethical Committee of the Balearic Islands (IB 4604/21 PI) and the Primary Care Research Committee of the Department of Majorca Primary Care (PI19/24). All participants are required to provide written informed consent and no study-related procedures will be performed until consent is obtained. The trial results will be published in peer-reviewed journals and presented at conferences.
Trial registration number ISRCTN10144646.
- Insomnia
- Primary Health Care
- Randomized Controlled Trial
- Protocols & guidelines
- Psychosocial Intervention
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Insomnia
- Primary Health Care
- Randomized Controlled Trial
- Protocols & guidelines
- Psychosocial Intervention
STRENGTHS AND LIMITATIONS OF THIS STUDY
If effective, the integration of cognitive–behavioural therapy for insomnia (CBT-i) into a primary care setting could decrease the administration of benzodiazepines to patients with insomnia.
The study will evaluate the effect of CBT-i under real-world conditions of clinical practice, thereby enhancing its external validity and relevance to everyday care.
Primary care physicians (PCPs) and primary care nurses (PCNs) will perform the multidisciplinary intervention.
A blinded final evaluation at 6 months and 12 months may mitigate ascertainment bias, but treatment contamination of the control group may decrease internal validity.
Difficulties in recruiting PCPs, PCNs and patients may limit the sample size and decrease the generalisability of the results.
Introduction
Insomnia is the most common sleep disorder and a very frequent pathology in the general population. A previous study in Spain estimated the prevalence of insomnia was 6.4%, and that 20.8% of people reported insomnia-related symptoms.1 The main problem reported by patients with insomnia is that it adversely impacts their daily activities.2 3 More specifically, patients with insomnia have a higher risk of chronic and acute health problems, a greater frequency of mood disorders (anxiety and depressive symptoms) and difficulties with concentration, memory, reasoning and problem-solving.4 5 Insomnia is also associated with an increased risk of traffic accidents6 and leads to numerous work-related problems, such as decreased productivity, increased accidents, lower job satisfaction, increased absenteeism,2 greater use of medications and increased consumption of alcohol.7 These patients have high direct costs due to their treatment for insomnia, and high indirect costs due to their increased use of other healthcare resources, increased accidents, decreased work productivity and the presence of comorbidities.8 9
Only 27%–52% of people with insomnia consult a professional about this problem,10 and their first visits are typically with primary care physicians (PCPs).11–13 PCPs most often treat these patients by prescribing hypnotic medications (benzodiazepines and Z drugs),14–17 recommending improvements in sleep hygiene and administration of herbal medicines (phytotherapy).18 19 Very few studies have analysed the roles of primary care nurses (PCNs) in helping these patients, but there is evidence that interventions by nurses are uncommon and mainly consist of providing advice on sleep hygiene and phytotherapy.20 21 However, PCPs and PCNs only rarely prescribe cognitive–behavioural therapy for insomnia (CBT-i), a non-pharmacological intervention that has proven benefits as a first-line treatment for persistent insomnia.13 22
CBT-i is a multicomponent intervention that focuses on cognitive and behavioural factors that contribute to the onset of sleep disorders. This therapy includes interventions that aim to improve sleep hygiene and often employs interventions to control different stimuli, sleep restriction therapy to decrease the time in bed while not sleeping, cognitive restructuring to develop more positive thoughts about sleep, paradoxical intention to decrease anxiety about sleep and various relaxation techniques. These interventions can be used independently or in combination.23 There is evidence that CBT-i is the most effective short-term and long-term treatment for insomnia.13 24 25 For example, it is more effective than usual care (UC) and more effective than medication when administered by psychologists and psychiatrists,24 even in patients with comorbidities.26 Therefore, many healthcare providers recommend CBT-i as the first-line treatment for insomnia, even if the proximal cause is unknown.27 However, because many insomniacs have limited access to mental health specialists, it is important to implement these interventions in a primary care setting, where most of these patients first seek treatment.28 It is also important to train primary care professionals in the use of certain components of CBT-i. Some studies have shown that educating family medicine residents to incorporate some components of CBT-i into their practices can help patients by providing them with important first-line treatment for their sleep problems.29
Nevertheless, very few studies have demonstrated the effectiveness of CBT-i when applied by PCPs and PCNs. Although there is evidence of fewer symptoms of insomnia after CBT-i, this treatment also has some shortcomings that are related to the duration and structure of the interventions. Therefore, more studies are needed to establish a ‘minimum effective dose’ (minimum duration and number of sessions) and to identify the most essential components of CBT-i needed to achieve a clinically significant result.30
Increasing the access to CBT-i in primary care settings is likely to be beneficial for patients with mild and moderate insomnia. However, there is a need to adapt training models and the methods of administering CTB-i to increase its acceptance in these settings and to convince patients about the benefits of this therapy.13
Following these recommendations, we carried out a pilot study to evaluate the feasibility and acceptability of a CBT-i intervention administered by PCPs and PCNs that was adapted from Morin’s theoretical model.23 The results of this pilot study indicated the need to address several key issues before beginning a large clinical trial31: (a) removing the cognitive restructuring component because PCPs do not feel comfortable administering this intervention; (b) providing additional training to primary care professionals to ensure they have the necessary skills; (c) increasing the treatment compliance of patients who have more severe insomnia and poorer quality of sleep and (d) decreasing the number of CBT-i sessions and providing greater flexibility to patients regarding their attendance at these sessions. We, therefore, designed the present study by focusing on these changes and evaluated the effectiveness and cost–utility of an adapted CBT-i intervention in a primary care setting whose aim is to improve the quality of sleep in patients with insomnia.
Our primary objective is to evaluate the effectiveness and cost–utility of a CBT-i intervention administered by PCPs and PCNs on sleep latency after 6 and 12 months compared with UC. The secondary objectives are to evaluate the effectiveness of the CBT-i intervention at 6 and 12 months on sleep quality, total sleep time, night awakening and the time between going to bed and falling asleep. We will also evaluate the safety of the intervention by examining its effects on anxiety and depression at 12 months. The current paper describes the design of the study.
Methods and analysis
Study design
This is a multicentre, two-arm, cluster-randomised controlled trial. Allocation will be performed at the level of treatment delivery (by PCPs and PCNs) to reduce bias from treatment contamination. The trial will be performed in Majorca Healthcare Centres (Spain). The intervention group will receive CBT-i and the control group will receive UC.
The Standard Protocol Items: Recommendations for Interventional Trials reporting guidelines have been followed to check protocol.32
Eligibility and recruitment of subjects
To recruit PCPs and PCNs, we will give a presentation describing the study in each healthcare centre of Mallorca Island. We will include patients who have scores of 8 or more on the Insomnia Severity Index (ISI), and additionally, whose insomnia interferes with daily functioning or is noticeable to others; sleep difficulties that cannot be explained by a medical condition or another mental or sleep-wake disorder; and a sleep problem that cannot be attributed to the use of recreational drugs or medications. Patients are eligible whether they do or do not use hypnotics. The exclusion criteria are secondary insomnia, such as diagnosed with sleep apnoea and receiving continuous positive airway pressure treatment, as recorded in the primary care electronic records, restless leg syndrome, parasomnia, or alterations of the circadian rhythm (eg, due to shift work), or use of a medication that could produce sleep alterations; any severe psychiatric disorder (psychosis, bipolar disorder and major depression); a previous suicide attempt; abuse of alcohol or drugs during the year before study onset; receipt of another CBT-i; prior diagnosis of dementia; any neurodegenerative or oncological disease with poor prognosis; any mental or physical incapacities that would prevent participation in interviews; acute or chronic pain secondary to a rheumatic disease or another untreated chronic disease; pregnancy or participation in a previous clinical trial at any of the participating health centres.
Based on consultations with family PCPs or PCNs, patients will be recruited using opportunistic inclusion, by the use of posters in the healthcare centres and by review of the electronic clinical records of the participating professionals. If a patient agrees to participate, then he or she will complete the ISI,33 a seven-item self-administered questionnaire that assesses the nature, severity and impact of insomnia. The usual recall period is ‘last month’. Three questions of the ISI assess specific insomnia problems: falling asleep, staying asleep and waking up too early. Four other questions evaluate sleep satisfaction, awareness of the sleep problem by others, stress caused by sleep problems and sleep problems that interfere with daytime functioning. A 5-point Likert scale is used to rate each item from 0 (no problem) to 4 (very severe problem), and the total score ranges from 0 to 28. The total score is interpreted as follows: absence of insomnia (0–7); subthreshold insomnia (8–14); moderate insomnia (15–21) and severe insomnia (22–28). We will only include patients with ISI scores of 8 or more, and who also score 3 (much) or 4 (very much) on the question about how sleep problems interfere with daily functioning and/or the question about how noticeable it is to others.
Eligible patients will sign an informed consent agreement, receive an appointment for a baseline evaluation (T0) and be informed of the allocation.
The privacy of patient data will be maintained before, during and after the trial. Only the professional who recruited them, the principal investigators and the external evaluator will have knowledge of their identity. A code has been assigned to process their data, which is known only to the principal investigators.
Sample size
We want to ensure that the clinical trial has adequate statistical power to detect a clinically significant decrease of at least 12 min in sleep latency in the CBT-i group relative to the UC group (two-tailed significance, alpha risk of 5% and beta risk of 20%). Assuming a 35% lost to follow-up and an SD of 22.6 min, we estimate that 86 subjects are needed in each arm.
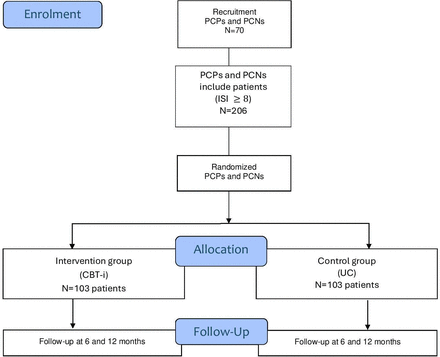
Each participating PCP or PCN will recruit three patients before randomisation, and we expect a 0.04 intraclass correlation coefficient between clusters, yielding a 1.20 cluster-design effect. Thus, the final targeted sample size is 206 patients (103 in each arm), and 70 professionals are needed (figure 1).
Participant flow diagram. CBT-i, CBT-i cognitive-behavioural therapy for insomnia; ISI, Insomnia Severity Index; PCNs, primary care nurses; PCPs, primary care physicians; UC, usual care.
Randomisation
Each participating PCP and PCN will send a list of included patients to the study coordinator. Then, a computer-generated random allocation procedure will be used to assign the PCPs and PCNs to one of the two arms. The PCPs and PCNs will be informed about allocation concealment and are, therefore, not blinded to the intervention. However, the main outcome assessment will be conducted by other participating healthcare professionals not involved in the study and blind to the allocation concealment.
Intervention
The CBT-i will be delivered by PCNs and PCPs with the active participation of the patients. The intervention consists of four individual structured sessions (three in 20 min consultations and one in a 10 min telephone call) that are separated by 2–3 weeks; patients taking a hypnotic medication will receive one additional session (table 1). The patients, PCNs and PCPs will agree on the therapeutic objectives before study’s onset. At the end of each session, the patient will have at-home tasks designed to help the patient achieve the objectives and learn the necessary techniques. Participating PCPs and PCNs will receive guidelines about the intervention. The patients will receive a handbook that contains a sleep diary for recording routines and times, and guidelines about sleep hygiene that include techniques to be used for relaxation and restricting time in bed. The participating PCPs and PCNs will register all relevant information that occurred during each session in the electronic clinical records.
Objectives, techniques and worksheets used in the CBT-i sessions
To standardise the intervention, the PCPs and PCNs in the intervention group will receive a 5-hour workshop prior to study onset. To develop the content and dynamics of this workshop, we considered suggestions from professionals who participated in the pilot study.31 This workshop will emphasise the conceptualisation of CBT-i, provide a deep review of each element of the intervention (the sleep diary, identification of sleep problems, control of stimuli, relaxation and techniques for restricting time in bed) and explain how to work with the patients to reach an agreement on the objectives. This training will be based on problem-solving from case reports.
Usual care
UC for insomnia patients in the primary care setting of Majorca was described in two previous studies published by our team. In particular, PCPs15 will treat insomnia with sleep hygiene counselling and prescription of benzodiazepines. PCNs will primarily use sleep hygiene counselling and phytotherapy.20 CBT-i was only very rarely used as UC. Using UC allows for a realistic comparison that reflects the standard clinical practice.
Evaluation
All included patients will be evaluated in a baseline interview (T0), at 6 months (T6) and at 12 months (T12). To guarantee blinding, the data collection notebook (DCN) for T0 will be separated from DCNs for T6 and T12.
Measures
Primary outcome measure
The primary outcome measure is sleep latency at 0, 6 and 12 months, which will be measured using the latency dimension of the Spanish-language version of the Pittsburgh Sleep Quality Index (PSQI).34 The PSQI is a 19-item self-administered questionnaire that assesses seven clinically relevant components of sleep quality during the previous month: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication and daytime dysfunction. Each component is rated from 0 to 3 points, based on the composite score from the frequencies of each disturbance; a score of ‘0’ corresponds to ‘not in the past month’ and a score of ‘3’ corresponds to ‘3 or more times per week’. The total score (the sum of the 7 component scores) ranges from 0 to 21, and a cut-off of 5 was used to discriminate good sleepers and poor sleepers. The European Drug Agency recommends evaluation of sleep latency to assess the effectiveness of insomnia treatments.35
Secondary assessments at 0 and 12 months
The following seven general measurements will be used as secondary assessments.
The Spanish-language version of the PSQI34 will be used to assess sleep efficiency, total sleep duration and severity (PSQI total score).
The Spanish-language version of the EuroQol 5 Dimensions (EQ-5D)36 with five dimensions (mobility, personal care, daily activities, pain/discomfort and anxiety/depression). Each dimension scores 1 (no problems) to 3 (a lot of problems). Also measures general health in the last 12 months through an analogical visual scale from 0 to 100 where 0 is the worst state of health and 100 is the best.
Direct healthcare expenditures will be calculated as the costs of medications, visits to the healthcare system (PCPs, PCNs, hospital and community emergency systems and hospital specialists), diagnostic and therapeutic procedures, and inpatient care.
Indirect costs will be calculated as costs related to the need for leave from work.
The use of hypnotic and antidepressant medications (self-declared and by the Spanish-language version of the Severity of Dependency Scale37) will be used to measure the use of benzodiazepines. This scale has five items (each scored from 0 to 3, with a total score of 0–15), and a higher score means greater dependency.
Safety issues and adverse effects will be measured. These are defined as any unfavourable effect, sign, symptom or disease that is reasonably associated with the CBT-i treatment, including tremors, anxiety, seizures, irritability and dizziness. Therapists will be asked to report these events to the research coordinator and ethical committee during the follow-up period. A separate safety committee will perform the analysis of safety using two scales. First, assessment of generalised anxiety disorder (GAD) will use a Spanish-language version of the GAD-7 questionnaire.38 This is a one-dimensional self-administered instrument that assesses the presence of symptoms of GAD, as described in the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV. The score of each item ranges from 0 (not at all) to 3 (nearly every day), and the total score ranges from 0 to 21. Based on the total score, the severity of GAD will be classified as minimal (0–4), mild (5–9), moderate (10–14) or serious (14–21). The Spanish-language version of the Patient Health Questionnaire-939 will also be administered. This scale has nine items that assess the presence and severity of depressive symptoms during the previous 2 weeks, and the score of each item ranges from 0 (not at all) to 3 (nearly every day).
Independent variables
We will record the following information for all patients: allocation group; demographic characteristics (age, sex, level of education, marital status, cohabitation status); anthropometric measurements (weight and height); chronic morbidities; use of alcohol, tobacco and other drugs; use of hypnotics and other medications.
Patient and public involvement
Individual interviews with four patients from the pilot study are used to guide the process of recruitment, the nature of the intervention and development of the patient booklet. These interviews will examine the most relevant objectives from different perspectives. Patients have suggested changes, primarily semantic, as some phrases in the homework book we provided confused them when reading it. Subsequently, these changes have been made.
Statistical analysis
The estimated effects will be determined using the 12-month results from the PSQI for sleep latency in the intervention and control groups. Generalised mixed linear random effect models will be used to account for clustering at the level of caregivers, with adjustments for baseline values. A p≤0.05 will be considered statistically significant.
All analyses will be conducted on an intention-to-treat basis, in that all initially randomised patients will be included in the analysis based on group assignment. The results will be reported according to the 2010 Consolidated Standards of Reporting Trials guidelines.40 Three subgroup analyses of effectiveness will be carried out for different patient groups: patients with a prescription for benzodiazepines or Z-drugs versus those without prescriptions; patients aged 65 years or older versus those younger than 65 years and males versus females. Descriptive analysis will be used to assess the significance of differences in the intervention and control group in baseline characteristics and will include calculations of means and/or proportions with CIs and SDs (to account for clustering).
The relative and absolute risk reduction, and the number needed to treat to prevent one patient from experiencing insomnia (score of 8 or more on the ISI) will be estimated. All estimates will include 95% CIs.
The health economic analysis will be conducted by calculating the incremental cost-effectiveness ratio (ICER) at 12 months from the perspective of the healthcare system. Data on the utilisation of all resources, including inpatient care, consultations with healthcare providers, medications and laboratory tests, will be systematically collected. Then, the EQ-5D scores will be used to measure these effects, and quality-adjusted life-years (QALYs) will be determined. The ICER will be calculated as the difference in mean costs between the two groups (C1–CT) divided by the difference in mean effects between the two groups (E1–ET).
A non-parametric bootstrap procedure will be employed to estimate the uncertainty associated with the estimated ICER. This procedure considers the skewness of cost data and the covariance of costs and QALYs. An alternative method, net-benefit regression, will also be used to control for potential confounding and to account for clustering. Cost-effectiveness acceptability curves will be presented to display statistical uncertainty. The safety of all interventions will be assessed using a per-protocol analysis, and the χ2 test will be used to compare adverse events.
Ethics and dissemination
This project was approved by the Ethical Committee of the Balearic Islands (IB 4604/21 PI) and the Primary Care Research Committee of the Department of Majorca Primary Care (PI19/24). All participants are required to provide written informed consent; no study-related procedures will be performed until consent is obtained. The trial results will be published in peer-reviewed journals and presented at conferences.
Modification of the protocol
If it is necessary to make any modifications to the protocol that may impact the conduct of the study, the potential benefits for patients or patient safety—including changes in study objectives, study design, patient population, sample sizes, study procedures or significant administrative aspects—will require a formal amendment to the protocol. Such amendment will be agreed on and approved by the Ethics Committee/IRB (Ethical Committee of the Balearic Islands) prior to implementation and notified to the health authorities in accordance with local regulations. Administrative changes to the protocol are minor corrections and/or clarifications that have no effect on the way the study is to be conducted.
Confidentiality
All study-related information will be stored securely at the study site. All participant information will be stored in locked file cabinets in areas with limited access. All data collection, process and administrative forms will be identified by a coded ID (identification) number only to maintain participant confidentiality. Informed consent forms will be stored separately from study records identified by code number. All local databases will be secured with password-protected access systems.
Intrastudy data sharing
Principal, coprincipal Investigators and statistician will be given access to the cleaned data sets. Project data sets will be housed in the research unit of Gerencia de Atención Primaria.
Discussion
Although insomnia is a highly prevalent sleep disorder and has a significant individual and economic impact, a review of the literature shows that PCPs and PCNs have little training in the treatment of these patients.15 19–21 In particular, very few PCPs and PCNs use CBT-i in their usual practice, even though this treatment has proven effectiveness.19 It is likely that these professionals are reluctant to administer CBT-i because of their high workload and their limited knowledge of this therapy.1 This study seeks to address some of the challenges in implementing conventional CBT-i by using a brief CBT-i intervention to make it feasible to conduct in a primary care setting.
A challenge of this study will be achieving the full sample size due to the difficulties in recruiting both professionals and patients. Many professionals experience a high workload, which can hinder their motivation to participate in clinical trials. Additionally, some participating professionals may lack the capacity to effectively recruit patients. A limitation is that some of the PCPs and PCNs assigned to the UC group might administer some kind of CBT-i (or some elements of CBT-i) leading to ‘contamination’ of the control group. Moreover, PCPs and PCNs who agree to participate in a study of CBT-i probably have higher motivations to help these patients than other primary care professionals. In any case, the presence of these complications would cause the UC group to have a better outcome, leading to a decreased apparent efficacy of the intervention.
The diagnosis of chronic insomnia disorder according to DSM 5, International Classification of Sleep Disorders (ICSD)-3 and International Statistical Classification of Diseases and Related Health Problems (ICD)-11 is solely based on taking a clinical history (anamnesis) and the patient’s self-reports of sleep-onset, sleep maintenance and early-morning awakening, accompanied by dissatisfaction with sleep and any daytime impairment, as long as the symptoms occur despite adequate sleep time and the opportunity to sleep in a comfortable environment.41 However, some of these symptoms, particularly dissatisfaction with sleep, rely on the subjective impressions and beliefs of the patients. Therefore, in this study, we have only included patients who report poor sleep and who also indicate that insomnia interferes with daily functioning or is noticeable to others.
There is no consensus regarding the best variable for measuring the efficacy of an insomnia treatment. The European Medicines Agency35 recommends improvements in sleep latency, duration, continuity, daytime functioning or quality of sleep as subjective endpoints, as key indicators of the efficacy of sleep-related treatments. In this study, sleep-onset latency was chosen as the primary objective to enable comparison with findings from other studies.
Current status of the study
Patient recruitment began on 24 November 2022 and will conclude on 15 June 2024. Results are expected to be available in June 2025, 12 months after the completion of recruitment.
Ethics statements
Patient consent for publication
Acknowledgments
We would like to thank Leighton Jones for his contribution to the final editing of this document, Alberto Aguilera for his drawing, which we used as the logo for this study, and Bartolomé Villalonga for his contribution to the homework book with his illustrations.
References
Footnotes
X @mtorrens, @beltordar, @SusanaGtorrente, @Caterina Vicens, @mcvidalth
Contributors MdMT-D is the guarantor for managing the trial and led the development and implementation of the training workshop for primary care physicians and nurses. She also contributed to the writing of the manuscript. IT-D is the guarantor for the conception of the study, drafted the study protocol, sought funding and ethical approval, managed the trial and developed the questionnaires, intervention materials and sleep hygiene leaflets. She also led the development and implementation of the training workshop for primary care physicians and nurses. Along with SGT, she serves as one of the principal investigators, with full access to all study data, and shared responsibility for the integrity of the data and accuracy of the analysis. She also contributed to the writing of the manuscript. ME is the guarantor for the conception of the study, drafted the protocol and sought funding and ethical approval. She also contributed to the writing of the manuscript. SGT is the guarantor for writing the study protocol, sought funding and ethical approval, managed the trial and led the development and implementation of the training workshop for primary care physicians and nurses. She shared the role of principal investigator with IT-D, with full access to all study data, and responsibility for data integrity and accuracy. CV is the guarantor for the conception of the study, drafted the protocol, sought funding and ethical approval, managed the trial and developed the questionnaires, intervention materials, and sleep hygiene leaflets. She also led the development and implementation of the training workshop for primary care physicians and nurses. AL is the guarantor of statistical analysis, contributed to drafting the study protocol and sought funding and ethical approval. She also contributed to the writing of the manuscript. PL is the guarantor for the development of the study protocol. She also led the development and implementation of the training workshop for primary care physicians and nurses. MRP-P is the guarantor for the development of the study protocol. She also led the development and implementation of the training workshop for primary care physicians and nurses. JIRM is the guarantor for the development of the study protocol. MCV-T is the guarantor for the development of the study protocol and developed the questionnaires, intervention materials and sleep hygiene leaflets. JM-X is the guarantor for the development of the study protocol. M-JS-R is the guarantor for the development of the study protocol. AIEA is the guarantor for the development of the study protocol and led the development and implementation of the training workshop for primary care physicians and nurses. All authors critically reviewed the manuscript, made contributions and approved the final version. All authors accepted full responsibility for the finished work and/or the conduct of the study, had access to the data and controlled the decision to publish.
Funding This work was supported by the Spanish Ministry of Economy and Competitiveness, Carlos III Institute (www.isciii.es), grant PI PI19/00029. Scholarships were cofinanced with FEDER funds from the European Union and supported by funding provided by Red 'Atención Primaria y Promoción de la Salud' (RICAPPS) RD21/0016/0009. Trial sponsor: 'Gerencia de Atención Primaria de Mallorca' (Balearic Islands).
Disclaimer This funding source and the study sponsor had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Author note I have used artificial intelligence to find an example of a cover letter and to correct some phrase in English.