Article Text
Abstract
Objectives Telemonitoring (TM) of home non-invasive ventilation (NIV) has been shown to facilitate home/outpatient therapy set-up. However, the impact of long-term TM on therapy dropouts, compliance and leak control has not yet been clearly determined. This study evaluated whether the NIV dropout rate was reduced by TM combined with remote patient support compared with a non-telemonitoring (NTM) pathway.
Design Retrospective cohort study.
Setting Data were obtained from all agencies of a single home care provider in France.
Participants Adults with chronic respiratory failure (n=659) who started nocturnal NIV between January 2017 and December 2019 and had ≥8 days of NIV therapy (51% male; mean age 68.5±13.8 years; 35.5% on long-term oxygen therapy) were included. The TM group included 275 patients who spent ≥80% of the follow-up using TM, and the NTM group included 384 patients who had 0 to ≤10 days of telemonitoring during follow-up.
Primary and secondary outcome measures The primary outcome was the rate of NIV dropouts at 1 year (ie, treatment discontinuation, excluding deaths). Secondary outcomes included therapy compliance and leaks.
Results 82 patients died during follow-up. Significantly fewer patients in the TM vs NTM group had dropped out of NIV therapy at 1 year (13% vs 34%; p<0.001). After adjustment for age, sex, NIV usage at 1-month follow-up and the main underlying respiratory disease, TM was significantly associated with a lower risk of dropout (HR 0.33, 95% CI 0.23 to 0.49; p<0.001). At 1, 4, 8 and 12 months, a greater proportion of patients in the TM vs NTM group had NIV usage of >4 hours/day and control of leaks.
Conclusions In patients starting home NIV, TM with home care provider first-line support was associated with a lower therapy dropout rate at 1 year, and better compliance and leak control, compared with standard follow-up.
- Telemedicine
- Pulmonary Disease, Chronic Obstructive
- Thoracic medicine
Data availability statement
No data are available. The datasets underlying the manuscript are not available due to privacy/ethical restrictions.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The high number of included patients and the unselected nature of the cohort provide real-life information about the home non-invasive ventilation (NIV) dropout rate and the feasibility and impact of telemonitoring (TM).
This is the first study to investigate the role of NIV TM in reducing dropout rates.
The dataset did not include information about the severity of underlying disease, comorbidities and correction of hypercapnia during NIV.
The TM and non-TM groups differed with respect to several baseline parameters.
Introduction
Chronic respiratory diseases have a significant impact on healthcare systems worldwide, with 7% of hospital admissions across Europe caused by lung diseases such as chronic obstructive pulmonary disease (COPD) and obesity-hypoventilation syndrome (OHS).1 2 Chronic respiratory failure (CRF) is an important complication and cause of death in people with COPD or OHS.3–5 Non-invasive mechanical ventilation (NIV) is a widely used and accepted treatment for the treatment of hypercapnic CRF, reducing hypercapnia, improving survival and quality of life, and reducing the need for hospitalisation.6–8 Data from the Eurovent survey, conducted in 16 European countries between 2001 and 2002, showed that >6.6 people per 100 000 experience CRF and require NIV.9
As with other therapies, effectiveness and tolerability are key factors in successfully achieving the desired clinical benefits of NIV therapy. There is currently limited data on patients who stop NIV therapy, and the dropout rate in cohort studies and randomised controlled trials varies widely, ranging from about 10% to 50% within 2 years of NIV initiation.10–15 Rates of NIV therapy dropout may be even higher in real-life settings.
Provision of home care support for NIV therapy varies widely between countries in Europe.16 In France, after inpatient initiation, home NIV is supported by a home care provider, who supplies the medical device and consumables, and provides training in NIV use and handling, and administrative support. Follow-up includes mandatory home visits every 2–4 months, during which the provider checks ventilator usage, supports the patient, performs technical maintenance and reports to the physician on compliance or technical issues, if any; there is also a 24/7 hotline available. With the growing use of home respiratory equipment and telemetry being available as an option for most home ventilators, integration of NIV telemonitoring (TM) and remote home care provider support, rather than disjointed and reactive follow-up, has been shown to improve the quality and effectiveness of NIV therapy.17–19
Our hypothesis was that telemonitoring and remote home care provider support would reduce the dropout rate from NIV by improving compliance and control of adverse effects. Therefore, this real-world cohort study investigated the dropout rate during the first year of NIV therapy in patients who were followed using TM and remote support compared with no telemonitoring (NTM).
Methods
Study population and data source
Eligible participants were adults (age ≥18 years) who started nocturnal home NIV between January 2017 and December 2019, used a non-life-support ventilator, received ≥8 days of NIV treatment and were followed-up by a single French home healthcare provider (Orkyn-Pharmadom, an Air Liquide Healthcare company). Patients were excluded if they had switched from NIV to continuous positive airway pressure (CPAP) or a life-support ventilator within the first year of treatment or if their data were invalid or missing.
The study was performed in accordance with French regulations, following the methodology MR-004 of the French Data Protection Authority (‘Commission Nationale Informatique et Liberté’ CNIL) concerning research that reuses previously collected data. Potential participants were provided with written information about the study and could decline the reuse of their personal data. Data usage and confidentiality were ensured in accordance with the CNIL MR-004 methodology. The study was registered on the national Health Data Hub Website (N°I52121510192019) and did not require investigational review board approval.
The study analysed data that had been prospectively collected by the home care provider as per routine practice for NIV home care services. This included patient demographic data (age, sex), area of residence (rural or urban), primary indication for NIV (COPD, OHS or other condition), respiratory support therapy characteristics (start and end date, type of ventilator, concomitant use of long-term oxygen therapy (LTOT)) and objective NIV device data (usage, unintentional leaks).
Patient and public involvement
Due to the retrospective nature of the analyses, patients and/or the public were not involved in the study design and study enrolment.
Study groups and provider follow-up
The TM group included patients who had remote transmission of NIV data activated during the first month after NIV initiation and were monitored using TM for ≥80% of their follow-up period (if follow-up was <1 year) or ≥292 cumulative days in the first year of therapy (if follow-up was ≥1 year). The NTM group had either no activation of the communication functionality of their NIV device or used TM for <10 days during the follow-up period. Participants who did not meet the criteria for inclusion in either the TM or NTM groups were excluded.
All participants received standard home NIV services as required by French regulation, including in-hospital NIV therapy initiation, a phone call from the provider technician on day 8 to ensure correct use of the NIV device and home visits at day 15 then every 2–4 months thereafter. At each home visit, NIV data were downloaded from the ventilator by the technician.
Patients in the TM group used a ventilator that had integrated telemetry (Lumis 150, ResMed). Daily remote transmission of NIV data (leaks, usage) started 1 week after therapy initiation, and data were processed through the Chronic Care Connect telemonitoring solution (Air Liquide Healthcare, Bagneux, France).18 This includes a CE-marked algorithm that generates alerts when specific criteria are met (if, over a period of 8 days, no data were received, mean NIV usage was ≤4 hours/day, and/or unintentional leaks were >24 L/min) plus a monitoring platform (with home care provider specialist nurses managing the alerts during working hours). Alert management (within two business days of the alert) included a nurse call to the patient and, if necessary, implementation of measures to reinforce compliance or resolve a technical issue.
Study outcomes
The primary outcome was the rate of dropout from home NIV in the first year after NIV initiation for reasons other than death. NIV dropout was defined as the cessation of an NIV order decided by the respiratory physician in charge of follow-up. Secondary outcomes were survival, NIV quality parameters (daily usage and unintentional leaks assessed at 1, 4, 8 and 12 months) and alert burden (in the TM group only). Alerts were collected and analysed from February 2018 onwards over a period of 12 months.
Statistical methods
Data are presented as number and percentage for qualitative variables, and mean±SD or median (IQR) for quantitative variables.
The rate of dropout from home NIV was estimated in both groups using the unadjusted Kaplan-Meier method over time (days) during the first year after NIV initiation; censoring took place at the date of death or at the end of follow-up.
The impact of TM on the NIV dropout rate was determined using univariate and multivariate Cox proportional hazards models. Univariate relationships between NIV dropout and each factor (TM status, patient sociodemographic and clinical characteristics [age, sex, underlying respiratory diseases, area of residence, usage of LTOT] and NIV quality parameters [daily usage, unintentional leaks] during the first month of usage) were evaluated using the log-rank test. Factors significantly associated with NIV dropout in the univariate analysis were then included in the multivariate analysis using a forward stepwise selection approach. The models included categorical variables and median age.
For each patient, median (IQR) values for daily NIV usage and unintentional leaks were calculated over the 30 days preceding the technician’s home visit. Values were compared between groups using the Wilcoxon test. The proportion of patients with high adherence (>4 hours/day) and/or no excessive unintentional leakage (≤24 L/min) was compared between groups using the x² test.
All analyses were performed using R 4.1.2 software (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as a two-sided p value of <0.05.
Results
Study population and baseline characteristics
A total of 659 patients were included (mean age 68.5±13.8 years; 51% male): 275 in the TM group and 384 in the NTM group (table 1). At baseline, the proportions of patients living in an urban area, with COPD or other condition, and using LTOT were significantly lower in the TM vs NTM group, while the proportions living in a rural area and with OHS were significantly higher. Telemetry started within the first week after NIV initiation (mean 3±6 days) for most patients in the TM group. During the first month of therapy, mean daily NIV usage was significantly higher, and unintentional leaks were significantly lower, in the TM vs NTM group (table 1).
Patient baseline characteristics, overall and by study group
NIV therapy dropouts during the first year (primary objective)
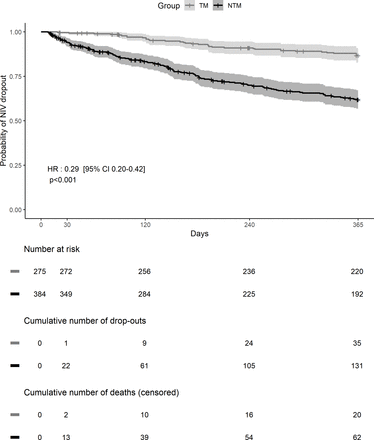
There were 136 NIV dropouts in the first year: 35/275 (12.7%) in the TM group and 131/384 (34.1%) in the NTM group (p<0.0001) (figure 1). In univariate analysis, older age, lower mean NIV usage at 1 month, lack of TM and underlying respiratory disease were significantly associated with increased NIV dropout risk. In contrast, use of LTOT, sex, unintentional leaks at 1 month and area of residence were not significantly associated with NIV dropout risk (figure 2). In the multivariate model adjusted for age, underlying respiratory disease, and NIV usage at 1 month, TM was significantly associated with lower dropout risk in the first year after NIV initiation compared with NTM (HR 0.33, 95% CI 0.23 to 0.49; p<0.001) (figure 3).
Kaplan-Meier curves showing the dropout rate in the telemonitoring (TM) and non-telemonitoring (NTM) groups over the first year of non-invasive ventilation (NIV) treatment. The shaded areas indicate the 95% CIs around the observed dropout rates. Censored events are denoted by tick marks on the curves.
Forest plots of univariate analysis for risk factors associated with non-invasive ventilation (NIV) dropout. Variables with a p value <0.05 on univariate analysis were included in the multivariate model. COPD, chronic obstructive pulmonary disease; LTOT, long-term oxygen therapy; NTM, non-telemonitoring group; OHS, obesity-hypoventilation syndrome; TM, telemonitoring group.
Forest plots of multivariate analysis for risk factors associated with non-invasive ventilation (NIV) dropout. Variables with a p value <0.05 on univariate analysis were included in the multivariate model. COPD, chronic obstructive pulmonary disease; LTOT, long-term oxygen therapy; NTM, non-telemonitoring group; OHS, obesity-hypoventilation syndrome; TM, telemonitoring group.
Survival
During the first year of NIV treatment, 82 patients died: 20/275 (7%) in the TM group and 62/384 (16%) in the NTM group (p<0.001). The subgroup of patients who died had a higher mean age than the overall study population (73.5±11.2 vs 68.5±13.8 years, respectively), but the mean age of deceased individuals was similar in the TM and NTM groups (73.3±12.0 and 73.6±11.0 years, respectively). The death rate was 12.0% in patients with COPD, 6.9% in patients with OHS and 16.5% in patients with other or unknown respiratory diseases.
NIV use and leaks
Median (IQR) NIV use increased over time in both the TM and NTM groups, and there was no significant between-group difference in device usage at 12 months (7.0 [5.1, 8.8] hours/day in the TM group and 7.0 [3.2, 8.9] hours/day in the NTM group; p=0.152). However, the proportion of patients with NIV use >4 hours/day was significantly higher in the TM group than in the NTM group at all time points (figure 4). Unintentional leaks were significantly better controlled in the TM vs NTM group (median (IQR) values at 12 months: 2.0 [1.0, 15.0] vs 7.5 [1.0, 40.7] L/min; p<0.001), and fewer patients in the TM vs NTM group had high leakage. The proportion of patients with high adherence (>4 hours/day) and without excessive leakage (≤24 L/min) was significantly higher in the TM vs NTM group at all time points (figure 4).
Proportion of patients with daily non-invasive ventilation (NIV) usage >4 hours (upper panel), unintentional leakage ≤24 L/min (middle panel) or both (lower panel) evaluated at 1, 4, 8 and 12 months. *p<0.05; **p<0.01; ***p<0.001 for the difference between the telemonitoring (TM) and non-telemonitoring (NTM) groups.
Telemonitoring alerts
In the TM group, approximately half of the patients had an alert during the first year of therapy. Home care provider nurses managed an average of 3.2 alerts per patient per year over the 12-month period. Of these, 33% were triggered for low NIV usage and 16% for excessive leakage (table 2).
Alerts generated during the 1 year follow-up in the telemonitoring group (n=132)
Discussion
In this retrospective real-life cohort of patients with CRF who started home NIV, the implementation of remote NIV TM (with alerts to detect poor compliance and excessive leakage) and home care provider support was associated with a significant reduction in the NIV dropout rate during the first year of treatment. Overall, there was a 67% decrease in the dropout risk between 1 month and 1 year with TM-based follow-up compared with standard follow-up. Other factors associated with NIV termination within 1 year were older age and low NIV usage in the first month, but we did not find any association between NIV dropout and the type of underlying respiratory disease or concomitant use of LTOT. The lower dropout rate observed in the TM group was associated with higher NIV usage and better control of unintentional leakage. This was achieved through the management of a mean of 3.2 alerts per patient, and only just over half of all patients had ≥1 alert.
The increasing number of people being managed with home respiratory care, improvements in ventilator technology, limited healthcare resources and the potential to reduce the carbon footprint of care has led to the proposal of novel approaches to home respiratory care management.20 21 Remote monitoring with automatic device data transmission (without the need for patient intervention) has been used most extensively in the treatment of obstructive sleep apnoea with CPAP and has more recently been introduced for home NIV.22 23 There is now sufficient evidence to support the use of telemonitoring for outpatient or home initiation of NIV.24 In contrast, the evidence base for the use of TM over longer periods is more limited, particularly, in relation to regular follow-up between physician or home care provider visits, especially, in patients with CRF at risk of clinical instability.25 Studies with a follow-up of >6 months suggested that TM was associated with a reduction in healthcare utilisation in patients with amyotrophic lateral sclerosis26 or COPD,13 and better NIV quality (ie, higher device usage and better leakage control) in patients with CRF not related to a neuromuscular disease.18 The only published randomised controlled trial comparing TM with standard NIV follow-up enrolled 148 patients with COPD across China and found that TM was associated with better health-related quality of life at 12 months and a significantly lower risk of readmission.19
In contrast, little attention to date has been paid to the impact of TM on therapy dropout rate, and what data that do exist are highly variable. In randomised controlled trials of patients with COPD patients using home NIV, dropout rates were reported to range from 9% to 33% at 1 year10 13–15 and reach up to 50% after 2 years.11 In the NTM group of our unselected patient population, the dropout rate in the first year of NIV therapy was 34.1%.
Our study presents the first evidence that TM combined with alerts and remote support of patients by a home care provider may significantly reduce the NIV dropout rate for reasons other than death. Our multivariate analysis suggested that TM and NIV usage of ≥4 hours per day in the first month of therapy had a significant positive effect on therapy adherence. Conversely, older age was identified as a risk factor for dropout in the first year, and the type of underlying respiratory disease was not found to be associated with dropout. These observations suggest that the primary factors contributing to dropout are not directly related to specific symptoms or aspects of a pathology but rather to tolerance, perception of benefit and therapy adherence.
Our findings are consistent with those of a single-centre case-control study that included consecutive patients who were non-adherent to NIV, who were then matched by the underlying cause of CRF with patients who had NIV adherence of ≥4 hours/day.27 The case-control study found that age >65 years, non-compliance with other therapies and previous hospitalisation with NIV use were independently associated with poor adherence and dropout.27 Furthermore, our data suggest that good compliance with NIV in the first weeks of therapy predicts continued usage at 1 year. This finding corroborates the results of a previous study using the same TM system, where the most significant predictors of successful NIV were NIV usage of ≥4 hours/day and control of leaks within the first 15 days after therapy initiation, and the majority of patients who discontinued NIV therapy had not achieved adequate compliance.18
Another objective of this study was to determine whether use of TM was associated with better NIV adherence and better control of unintentional leakage. Previous observational cohort studies have shown that TM allows the optimisation of ventilation delivery and patient compliance.18 28 Furthermore, in the only randomised controlled trial comparing TM with standard NIV follow-up, average NIV usage was 47 min longer at 6 months and 27 min longer at 1 year with TM vs standard follow-up.19 Similarly, we found that the proportion of patients with daily NIV usage ≥4 hours/day and controlled leakage increased over time in the TM group but plateaued in the NTM group. Indeed, NIV compliance increased from initiation to 1 year in most patients. Conversely, excessive leaks, which are typically controlled in almost all patients at the beginning of the treatment, can occur in some patients during follow-up. This trend may be due to the higher frequency of home care provider and medical visits in the first 4 months after NIV initiation. Therefore, the use of TM and remote support seems to be useful for monitoring and controlling excessive leakage during longer-term follow-up.
In the current study, the 1-year mortality rate was significantly lower in the TM group than in the NTM group (7% vs 16%). Previous studies have reported that NIV adherence >4 hours/day was associated with lower mortality than lower levels of adherence.29 However, we were unable to determine associations between use of TM and mortality during NIV therapy due to the retrospective nature of the study and the absence of a full set of data from each participant’s medical records.
Strengths of this study include the sample size and the unselected nature of the cohort, which provides real-life information about the dropout rate from NIV and the feasibility of TM. However, the real-life, retrospective design of the study also comes with some limitations that should be considered when interpreting the results. First, we did not have any medical data that allowed us to determine the severity of the underlying respiratory disease or the presence of comorbidities; data on arterial blood gas analysis (and therefore, the correction of hypercapnia during NIV) and health-related quality of life were also missing. In addition, there were several baseline differences in characteristics between the TM and NTM groups. However, several of these variables (age, underlying disease and use of LTOT) were included in the multivariate analysis. Another limitation is that reasons for the non-use of TM in patients from the NTM group, such as patient motivation, were not captured. It was assumed that factors such as patient motivation were likely to be well balanced between the two groups because the decision to use or not use TM was made by the physician or home care provider rather than by the patient. Importantly, in our study, all patients received similar home care NIV services and NIV data were collected in an identical manner via data downloaded from the ventilator during the technician visit. Nevertheless, the possibility remains that differences in the type of ventilator, ventilatory settings or interface might have influenced the results. In addition, the presence of a healthy user bias in the TM group might have impacted on our findings. Finally, our data might not be generalisable to all patients requiring home NIV and all geographical locations because the approach taken to standard follow-up by the home care provider is specified in French regulations, and practices elsewhere may differ.
In conclusion, this study provides the first evidence that the use of TM might reduce the 1-year dropout rate from home NIV, along with improving compliance and leak control. Future prospective, controlled, comparative studies are needed to corroborate our findings. Furthermore, because the benefits of NIV cease when therapy is discontinued, and patients with COPD have better clinical outcomes with vs without NIV,30 additional studies are needed to determine the impact of NIV dropout on survival, clinical outcomes and healthcare consumption.
Data availability statement
No data are available. The datasets underlying the manuscript are not available due to privacy/ethical restrictions.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by health data hub I52121510192019. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors would like to thank Orkyn' Pharmadom for subsidising this study and for providing medical-technical support for the development of the study and regulatory protocol for the identification and good understanding of the nomenclature of the medical devices concerned. The authors thank Maria Pini (Alira Health, Paris, France) for the assistance in writing and editing the manuscript.
References
Footnotes
Contributors RLM as the guarantor accepts full responsibility for the work and the conduct of the study, had access to the data and controlled the decision to publish. RLM, CGG and FK conceived the study and oversaw overall direction and planning. RLM, CGG, MG, FK and MR designed the model and the computational framework. MR and ACM performed the statistical analysis. RLM, CGG, JBT, MG and FK drafted the manuscript with input from all authors. FG and WT critically reviewed the manuscript.
Funding The study was funded by Pharmadom-Orkyn, a subsidiary of Air Liquide Healthcare. Grant no: NA.
Competing interests RLM declares receipt of personal fees from Orkyn-Pharmadom, CGG declares receipt of personal fees from Orkyn-Pharmadom. FK is an employee of Pharmadom-Orkyn. JT is an employee of Air Liquide Healthcare. ACM and MR are employees of Alira Health. FG declares receipt of personal fees from Air Liquide Sante, Asten Sante, Inspire, Bioprojet, ResMed and Sefam, payment for presentations from Asten Sante, Bioprojet, Cidelec, Jazz Pharmaceuticals, Philips Respironics, and ResMed, and non-financial support from Asten Sante. WT declares payment for presentations from Bioprojet and Astra Zeneca and non-financial support from Asten Sante. The authors perceive that these disclosures pose no academic conflict for this study.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.