Article Text
Abstract
Objective Zanubrutinib is a second-generation Bruton’s tyrosine kinase inhibitor that has been approved for the treatment of several B cell malignancies. The aim of this study was to evaluate adverse events (AEs) associated with zanubrutinib based on the real-world data.
Design A disproportionality analysis was performed to identify the potential zanubrutinib-related AEs.
Setting The Food and Drug Administration AE Reporting System database from the fourth quarter of 2019 to the third quarter of 2023.
Main outcome measures The results of the disproportionality analyses were presented as reported ORs (RORs). When the lower limit of the 95% CI for the ROR is greater than 1 and the number of AE reports is≥3, it indicates that the preferred term (PT) may be a positive AE signal.
Results A total of 846 AE reports with zanubrutinib as the primary suspect drug were obtained, with 2826 AEs. A total of 74 positive PT signals were detected across 18 system organ classes (SOCs). The most significant signal for SOC was ‘blood and lymphatic system disorders’ (ROR=2.8, 95% CI 2.3 to 3.3), while the most significant signal for PT was ‘haemorrhage subcutaneous’ (ROR=190.8, 95% CI 128.0 to 284.5). 13 unexpected off-label AEs were also observed, such as abnormal hair texture, skin discolouration, hypernatraemia, pericardial effusion and hypersomnia. The median time to onset of AEs associated with zanubrutinib was 51 days (IQR 13–192 days) and was consistent with the early failure model. In comparison with zanubrutinib monotherapy, the combination of zanubrutinib and rituximab therapy was linked to a higher risk of specific AEs, including myelosuppression, pneumonia, leucopenia, thrombocytopenia, abdominal pain, anaemia, pancytopenia and respiratory failure. Furthermore, the combination of zanubrutinib and chemotherapy increased the risk of several severe AEs, such as cardiac arrest, elevated blood lactate dehydrogenase levels and pancytopenia.
Conclusions The results of the analysis provided valuable insights into the safety profile of zanubrutinib-treated patients, which was helpful for clinical monitoring and identifying potential AEs related to zanubrutinib.
- Adverse events
- Clinical Pharmacology
- Health and safety
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The present study employs a disproportionality analysis to identify potential adverse events (AEs) associated with zanubrutinib, using the Food and Drug Administration Adverse Event Reporting System (FAERS).
Reported ORs and their corresponding 95% CIs were employed to identify the potential signals.
The FAERS database is a self-reporting system that may have some limitations, such as incomplete information and awareness bias.
The disproportionality analysis indicated a statistical correlation but did not establish a definitive causal relationship between the target drug and the specific AEs.
Induction
Bruton’s tyrosine kinase (BTK) is a non-receptor tyrosine kinase that is a member of the Tec (tyrosine kinase expressed in hepatocellular carcinoma) kinase family.1 2 BTK is mainly expressed in B lymphocytes, myeloid cells and platelets.3 BTK is a vital signalling molecule in the B cell receptor pathway and plays a crucial role in B cell differentiation, proliferation and survival.2 The treatment of B cell malignancies has been greatly improved by the development of the inhibition of BTK. Since the approval of the first BTK inhibitor, ibrutinib, by the US Food and Drug Administration (FDA) in 2013, a variety of BTK inhibitors have been used clinically to treat patients with B cell malignancies over the past decade and have demonstrated significant efficacy.4–9
Zanubrutinib is a selective second-generation oral BTK inhibitor that covalently and irreversibly binds to Cys-481 in the BTK ATP binding site.10 By optimising the molecular structure, zanubrutinib improves BTK target selectivity and minimises off-target binding, resulting in more precise and sustained BTK inhibition.10 11 Zanubrutinib was first approved by the FDA in November 2019 to treat patients with relapsed/refractory (R/R) mantle cell lymphoma (MCL).10 The approval was based on the results of two studies: phase I/II study BGB-3111-AU-003 (NCT02343120) and phase II study BGB-3111-206 (NCT0320697). The trial NCT02343120 recruited 32 patients who had previously received treatment for MCL. The study achieved an overall response rate (ORR) of 84%, with a complete response (CR) rate of 29.7%.12 In the phase II trial, 72 of 86 patients (84%) achieved an objective response, with 59 (68.6%) of them achieving a CR.13 The median duration of response and progression-free survival (PFS) was 19.5 months and 22.1 months, respectively. Zanubrutinib was approved by the FDA in August 2021 for the treatment of Waldenström’s macroglobulinaemia (WM) and in September 2021 for marginal zone lymphoma (MZL).11 In January 2023, zanubrutinib was granted FDA approval for the treatment of chronic lymphocytic leukaemia (CLL) and small lymphocytic lymphoma (SLL) based on the results of the ALPINE and SEQUOIA trials. The ALPINE study, an open-label, multicentre, randomised, phase III clinical trial evaluating the efficacy and safety of zanubrutinib versus ibrutinib in 652 patients with R/R CLL/SLL, showed that zanubrutinib was superior to ibrutinib in terms of ORR and PFS.14 The SEQUOIA study, which randomised patients with previously untreated CLL/SLL to zanubrutinib or bendamustine plus rituximab, showed a significant improvement in PFS with zanubrutinib compared with bendamustine–rituximab.15
Although BTK inhibitors have shown impressive efficacy and activity, they still require careful attention to safety and monitoring for some unique toxic reactions.16 Pooled data from six zanubrutinib monotherapy trials involving 779 patients showed that 98% of patients experienced at least one adverse event (AE).17 The most common non-haematological AEs were upper respiratory tract infection, rash, bruising, musculoskeletal pain, diarrhoea, cough and pneumonia, urinary tract infection, fatigue, haematuria, constipation, headache, fever, hypertension and nausea. However, clinical trials are conducted under strict limitations, and AEs of zanubrutinib observed in clinical trials may not fully reflect all AEs observed in clinical practice. Therefore, a comprehensive assessment of the postmarketing safety of zanubrutinib based on the real-world data is needed.
The FDA Adverse Event Reporting System (FAERS) is a free database of spontaneous AE reports submitted by manufacturers, healthcare professionals, individual patients and others. FAERS is widely used in signal mining studies of AEs, which can effectively monitor and evaluate the postmarketing safety of drugs.18 In the present study, we used data mining of FAERS to retrospectively identify and investigate the signals of zanubrutinib-associated ADRs.
Methods
Patient and public involvement
Patients and the public were not involved in our study.
Data sources and cleaning
This disproportionality analysis was performed to analyse zanubrutinib-related AEs using data from the FAERS database. Data ranging from the fourth quarter of 2019 (when zanubrutinib was approved by the FDA) to the third quarter of 2023 were selected for analysis. FAERS data are composed of seven datasets: demographic and administrative information (DEMO), drug information (DRUG), AE information (REAC), drug therapy start dates and end dates (THER), indications for the reported drugs (INDI), patient outcomes information (OUCT) and reported sources (RPSR). PRIMARYID linked the same AE report across different datasets. Data units from different seasons were combined using R software. Prior to statistical analysis, we performed deduplication based on the following standards: if the PRIMARYIDs were identical, the most recent FDA_DT was chosen; if both the PRIMARYIDs and FDA_DTs were identical, the lower PRIMARYIDs were deleted.19
AE identification and mining
Various drug names, including ‘zanubrutinib’, ‘BGB 3111’ and ‘Brukinsa’, were used in the search for zanubrutinib. In the FAERS database, there are four codes for the role of drugs in reported AEs: Primary suspect (PS), secondary suspect (SS), concomitant (C) and interacting (I). Only reports that document zanubrutinib as the role code of PS were selected for analysis to improve accuracy. AE reports in the FAERS database are coded using preferred terms (PTs) from the Medical Dictionary for Regulatory Activities (MedDRA). For the present analysis, the PTs and system organ classes (SOCs) were categorised according to MedDRA version 26.1. The European Union Drug Regulating Authorities Pharmacovigilance of Important Medical Events (IMEs) was used to evaluate serious AEs. Additionally, clinical characteristics, such as gender, age, outcomes of AEs, country of report and reporter occupation, were gathered.
A disproportionality signal occurs when the reported incidences of AEs for a targeted drug are higher than those in the background data. Reporting ORs (RORs) were used in our analysis to identify potential signals that may indicate an increased risk of drug-associated AEs for zanubrutinib. The ROR was calculated using a 2×2 contingency table that contrasted the reported event counts for the target medication with those of the other background drugs.20 The calculation formula for ROR is shown in online supplemental table S1. The magnitude of the ROR value represents the strength of the association between the reported drug and specific AE. The signal of PT was considered positive if the lower limit of the 95% CI for ROR was greater than 1 and the number of AE reports was≥3.20 PTs related to the progression of disease, medication errors and surgical operations were excluded.
Supplemental material
Subgroup analysis
Subgroup analyses were conducted to investigate the correlation between zanubrutinib and AEs within defined subgroups, classified according to indication and geographical region. The currently approved indications for zanubrutinib included MCL, CLL/SLL, WM, MZL and follicular lymphoma (FL). Owing to the limited number of cases of MZL and FL in our dataset, the subgroup analysis by indication was primarily focused on MCL, CLL/SLL and WM. With regard to regional subgroups, distinct analyses were performed for the United States and China, as these two countries constituted the largest number of cases in our dataset.
Time-to-onset analysis
The time-to-onset of AEs was calculated by subtracting the zanubrutinib initiation date (START_DT) from the AE onset date (EVENT_DT). AE reports with incomplete dates, no dates and incorrectly formatted dates were excluded. The time-to-onset was described using the Weibull shape parameter test and the proportion of events by time. The Weibull distribution is a continuous probability distribution that is described by a scale parameter (α) and a shape parameter (β). The time-to-onset analysis predicted the risk of AEs over time based on the shape parameter. The predicted results were classified into three categories: ‘early failure’, ‘random failure’ or ‘wear-out failure’. If both the shape parameter and its 95% CI were less than 1, the risk of AE is estimated to decrease over time, which is referred to as early failure. If the shape parameter is equal to or close to 1 and its 95% CI encompasses 1, the risk of AE is considered not to change over time, which is referred to as ‘random failure’. If both the shape parameter and its 95% CI were above 1, the risk of AE is predicted to increase gradually, which is referred to as ‘wear-out failure’.21
Drug combination analysis
The safety of zanubrutinib in combination with other drugs was also investigated. First, the drugs used in combination were retrieved from all reports that included zanubrutinib (PS, SS, I and C) and ranked according to the number of cases of combination. Preliminary results showed that the drug with the highest number of combinations with zanubrutinib was rituximab. Further disproportionality analyses were then performed on the drugs with the highest number of combination cases. In the disproportionality analyses for combination therapy, target drug cases were defined as zanubrutinib (PS)+rituximab (any role code) or rituximab (PS)+zanubrutinib (any role code), and background data were defined as zanubrutinib (PS) without rituximab. When the ROR signal was positive and p-value<0.05 (χ2 test), this indicated that the combination therapy was more likely to cause a specific AE than zanubrutinib monotherapy.22 The second most common drug combined with zanubrutinib was cyclophosphamide. Therefore, a further analysis was performed to explore the risk of AEs associated with the combination of zanubrutinib and chemotherapy.
R software version 4.3.2 was used for all data cleaning, mining, statistical analyses and graphs.
Result
Population characteristics
A total of 7 575 864 AE reports were recorded in the FAERS database between October 2019 and September 2023 (online supplemental figure S1). After removing duplicates, there were 846 AE reports associated with zanubrutinib as the PS, documenting a total of 2826 zanubrutinib-related AEs. Patient characteristics are summarised in table 1. In the included cases, hospitalisation—initial or prolonged—was the most common severe outcome, accounting for 25.7% (218/848). AEs with zanubrutinib resulted in 64 deaths (7.5%). Approximately 60% of the reports were submitted by healthcare professionals, such as physicians (33.0%), pharmacists (11.2%) and other healthcare professionals (14.4%). The majority of AEs were reported from the United States (61.7%), followed by China (16.6%).
Characteristics of reports associated with zanubrutinib
A total of 166 cases were reported in the FAERS for the combination of zanubrutinib and rituximab, with either zanubrutinib or rituximab as the PS. The most frequent severe outcome of AEs remained hospitalisation—initial or prolonged. The majority of the reports (62.0%) originated from China. Additionally, there were 78 reports associated with the combination of zanubrutinib and chemotherapy.
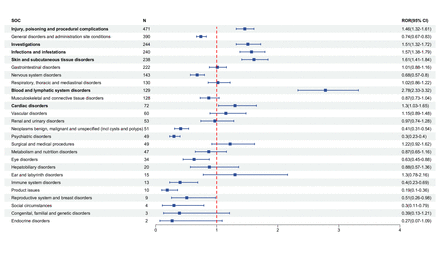
Signal of system organ classifications
AEs induced by zanubrutinib were found in 26 different organ systems (figure 1). Among them, six significant SOCs were identified, including ‘blood and lymphatic system disorders’, ‘skin and subcutaneous tissue disorders’, ‘infections and infestations’, ‘investigations’, ‘injury, poisoning and procedural complications’ and ‘cardiac disorders’.
Forest plot of the adverse events for zanubrutinib at the SOC level. ROR, reporting OR; SOC, system organ class.
Signal of PTs
A total of 74 positive PT signals belonging to 18 SOCs were detected in our analysis. Several positive signals that may be associated with lymphoma complications, such as ‘blood lactate dehydrogenase increased’, ‘splenomegaly’, ‘blood immunoglobulin M increased’ and ‘lymphadenopathy’, were not taken into account. The top 25 AEs with the highest ROR for zanubrutinib are displayed in figure 2, and the full list of positive PT signals based on the SOCs is listed in online supplemental table S2. The strongest AE signal was the PT of ‘haemorrhage subcutaneous’ (ROR=190.8, 95% CI 128.0 to 284.5), followed by ‘penile haemorrhage’ (ROR=112.6, 95% CI 35.9 to 352.6). Most of the positive signals were reported in the previous clinical studies or listed on the label for zanubrutinib. Nevertheless, 13 positive signals, such as skin discolouration, abnormal hair texture, hypernatraemia, pericardial effusion and hypersomnia, were not mentioned on the label.
Forest plot of the top 25 adverse event risk signals for zanubrutinib. PT, preferred term; ROR, reported OR.
In addition, 20 of the 74 positive PT signals were considered serious AEs according to the IME list (online supplemental figure S2). The largest number of cases occurred in myelosuppression, with 39 reported cases.
Results for subgroup analysis
The results of the indication-based subgroup disproportionate analysis are presented in online supplemental table S3. Across the MCL, CLL/SLL and WM subgroups, the ROR for the AE of ‘haemorrhage subcutaneous’ was found to be consistently the highest, with values of 32.2 (95% CI 9.4 to 110.3), 41.3 (95% CI 19.0 to 89.6) and 46.6 (95% CI 12.3 to 175.9), respectively. The most frequently reported AE in the MCL subgroup was dyspnoea, with 11 cases, in the CLL/SLL subgroup was a contusion, with 21 cases, and in the WM subgroup was rash, with 18 cases.
In the subgroup analysis of the US population, a total of 68 PTs exhibited positive signals (online supplemental table S4). Notably, ‘haemorrhage subcutaneous’ demonstrated the strongest signal with an ROR of 601.7 (95% CI 369.4 to 980.3). This was followed by ‘penile haemorrhage’ (ROR=176.1, 95% CI 55.7 to 556.1) and ‘petechiae’ (ROR=87.7, 95% CI 53.4 to 143.9).
In the Chinese population, the highest signal PT was ‘haemolysis’ (ROR=44.8, 95% CI 13.8 to 145.0), followed by ‘hepatitis B’ (ROR=40.0, 95% CI 12.4 to 129.1) and ‘tumour lysis syndrome’ (ROR=31.3, 95% CI 9.8 to 100.3).
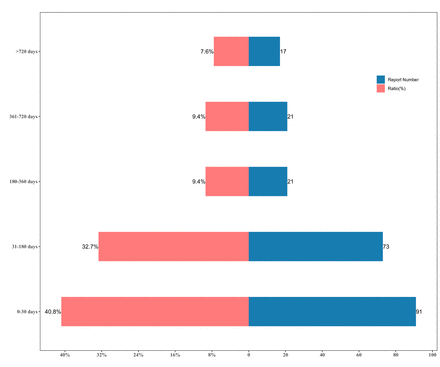
Results for the time-to-onset analysis
After excluding reports with incomplete dates, a total of 223 reports were included in the time-to-onset analysis, with a median onset time of 51 days (IQR 13–192). Out of the 223 reports, 91 (40.8%) occurred during the first month of zanubrutinib treatment, and a total of 164 (73.5%) occurred within 6 months of the initial dose (figure 3). A scale parameter of 123.7 (95% CI 95.0 to 152.4) and a shape parameter of 0.60 (95% CI 0.54 to 0.66) were obtained when fitting the time-to-onset to the Weibull distribution. It indicated that the risk of AEs associated with zanubrutinib should be referred to as 'early failure', and that the likelihood of experiencing AEs decreases over time.
Time-to-onset of zanubrutinib-related adverse events.
Results for the combination analysis
The five most commonly combined drugs with zanubrutinib in all reports from Q4 2019 to Q3 2023 in the FAERS database were rituximab (n=373), cyclophosphamide (n=231), prednisone (n=216), obinutuzumab (n=205) and vincristine (n=114). A disproportionality analysis was conducted to investigate the impact of coadministering rituximab on the safety profile of zanubrutinib. The results showed that 10 AEs (figure 4), such as myelosuppression, pneumonia, leucopenia and platelet count decreased, may be more likely to occur in patients treated with rituximab+zanubrutinib than in patients treated with zanubrutinib alone. Furthermore, the analysis of zanubrutinib combined with chemotherapy revealed that nine AEs, such as cardiac arrest, increased blood lactate dehydrogenase levels and pancytopenia, were at a higher risk of occurrence in the patient group receiving chemotherapy plus zanubrutinib than in those on zanubrutinib monotherapy.
Volcano plots of the difference in PT signals for the combination analysis. A: Zanubrutinib+rituximab versus zanubrutinib alone. B: Zanubrutinib+chemotherapy versus zanubrutinib alone. PT, preferred term; ROR, reported OR.
Discussion
BTK inhibitors have demonstrated superior clinical efficacy and tolerability in patients with B cell malignancies in comparison with standard chemotherapy and immunotherapy regimens.23 The AEs related to BTK inhibitors were mostly classified as grades 1 and 2, with a low frequency of grade ≥3 AEs.24 The rate of treatment termination due to AEs was relatively low. The first-generation BTK inhibitor ibrutinib displayed significant off-target effects. It inhibited other kinases non-specifically and bound to other signalling channel proteins, leading to a range of AEs. In contrast, zanubrutinib was a next-generation BTK inhibitor that had improved specificity and off-target effects, resulting in a lower incidence of treatment-related AEs.8 9 Nonetheless, patients treated with zanubrutinib may experience unique AEs that require close monitoring to ensure optimal efficacy.
A comprehensive disproportionality analysis of the safety profile of zanubrutinib was conducted based on postmarketing data from the FAERS database. In the almost 4 years since zanubrutinib was marketed, the FAERS database has documented 846 AE reports where zanubrutinib was the PS. A total of 74 positive PT signals were identified, which were involved in 18 of the SOCs.
The disproportionality analysis revealed that the AEs with the most significant signal at the SOC levels were related to 'blood and lymphatic system disorders'. Haematological toxicity, including neutropenia, thrombocytopenia and anaemia, was one of the most common AEs associated with zanubrutinib. Neutropenia was one of the few AEs that occurred more frequently with zanubrutinib than with ibrutinib (29% vs 13%; HR, 2.18; 95% CI, 1.15 to 4.12).9 The various complex mechanisms of immune dysregulation resulting from B lymphocytoma may contribute to the haematological toxicity.23 Severe haematological toxicity may lead to dose adjustment or discontinuation of zanubrutinib therapy.8 9 The most common cause of zanubrutinib dose reductions is neutropenia.17 Jiang et al25 developed an XGBoost model to predict the severe haematological toxicity of BTK inhibitors. The XGBoost model was constructed based on ten parameters: leucocytes, neutrophils, erythrocytes, platelets, fibrinogen, total albumin, aspartate aminotransferase, lactate dehydrogenase, gender and the type of BTK inhibitor. Among them, lactate dehydrogenase, neutrophils, BTK inhibitor (ibrutinib) and gender (female) were positively correlated with the outcome, while other factors were negatively correlated with the outcome. The XGBoost model is available online for clinical use.
Of the 74 positive PT signals we obtained, up to 18 were associated with haemorrhages, such as eye haemorrhage, haematemesis, subdural haematoma, haemarthrosis, haemorrhage intract, haematuria, penile haemorrhage, ecchymosis and skin haemorrhage. Bleeding was a frequently observed AE in patients treated with zanubrutinib, and the majority of these AEs were mild (grade ≤2).8 17 Clinical studies have shown that bleeding events of any grade occurred in 4.4%–66.0% of patients treated with zanubrutinib, with major bleeding occurring in 0.3%–3.5%.8 9 17 26 Bleeding was more likely to occur in patients≥75 years.17 Patients who suffered a bleeding event of grade ≥3 needed to discontinue zanubrutinib permanently unless the risk of rebleeding was deemed acceptable.23 Studies have shown an increased risk of bleeding when BTK inhibitors are combined with anticoagulants or antiplatelet agents.23
Studies on the pathophysiology of zanubrutinib-associated bleeding are limited. Currently, the mechanisms of haemorrhage associated with BTK inhibitors are mainly explored based on ibrutinib.3 27 It has been suggested that the risk of bleeding associated with BTK inhibitors may be due to both on- and off-target effects. BTK and TEC interfered with collagen-induced platelet activation by regulating the platelet transmembrane receptors, including the platelet glycoprotein VI (GPVI) and the C-type lectin-like receptor 2 (CLEC-2). GPVI is the main signalling receptor for collagen. The binding of collagen to GPVI triggers the platelet activation cascade.28 CLEC-2 activates signal transduction via tyrosine phosphorylation of a single YXXL motif in its cytoplasmic tail, which triggers the platelet activation cascade.29 BTK inhibitors irreversibly inhibit the BTK and TEC leading to the inhibition of GPVI- and CLEC-2-mediated platelet activation.3 27 In addition to GPVI and CLEC-2 signalling, inhibition of the GPIb and αIIbβ3-integrin pathways may also contribute to bleeding caused by BTK inhibitors.3 16
Patients treated with zanubrutinib were at high risk of infection due to immunosuppression. The infectious event with the most cases reported in the FAERS database was urinary tract infection (n=19; ROR 2.4; 95% CI 1.6 to 3.8). Opportunistic infections, including fungal pneumonia (ROR=23.7, 95% CI 10.6 to 53.0), cryptococcal meningitis (ROR=40.1, 95% CI 12.9 to 124.9) and pneumocystis carinii pneumonia (ROR=15.5, 95% CI 1.8 to 17.2), were detected. The pooled safety analysis revealed that infections had the highest incidence of AEs, with a 76% occurrence rate.17 Additionally, serious infections were reported in 27% of cases. The ALPINE trial showed that infectious events were the most common AEs leading to discontinuation.8 The mechanism of increased susceptibility to infection is complex, primarily involving the effect of BTK inhibitors on the immune system.26 BTK played a crucial role in detecting a broad spectrum of microbes via various toll-like receptors.30 BTK inhibitors may interfere with the sensing of pathogens. In addition, neutrophils are a crucial component of the human immune system, serving as a key defender against pathogenic pathogens. However, neutropenia is a common AE experienced by patients receiving zanubrutinib.
Unexpected and significant PT signals, such as abnormal hair texture, skin discolouration, hypernatremia, pericardial effusion, hypersomnia, intestinal perforation and blood iron decrease, were detected in our analysis. All of the unexpected and novel AEs need to be further confirmed in future studies and require vigilance in clinical practice. Hair changes were thought to be one of the skin toxicities of ibrutinib but have not been reported in zanubrutinib-treated patients. A meta-analysis of 32 clinical trials evaluated the dermatological toxicity of ibrutinib.31 Among the 32 clinical trials included, two of the phase II clinical studies for the treatment of CLL/SLL reported hair changes associated with ibrutinib (7.9%; 95% CI, 0.0 to 21.3%). A pharmacovigilance analysis of ibrutinib and acalabrutinib using the FAERS database identified 84 cases of ibrutinib-associated hair changes by December 2021 (ROR=108.7, 95% CL 85.0 to 139.1).32 However, no positive signal was found for acalabrutinib. The proteins in the keratinocytes of the hair contained an abundance of sulfur-containing amino acids that formed disulide bonds, which were important for the tensile strength and structural integrity of the hair.33 The covalent binding of the BTK inhibitors to cysteine residues in the BTK active site disrupted the disulide bond between cysteine residues, which may lead to hair changes.34 Skin discolouration, another unexpected dermatological toxicity that we detected, manifested primarily as abnormal skin pigmentation. However, it was not detected in either ibrutinib or acalabrutinib.31 32 34
Our analysis revealed that the median time-to-onset of all AEs associated with zanubrutinib was 51 days (IQR 13–192), with 73.5% of cases occurring within 6 months of exposure to the drug. This onset time appears to be shorter than that of most AEs reported in clinical studies. Clinical studies have shown that bleeding events, infectious events, neutropenia, thrombocytopenia, anaemia and atrial fibrillation had a median time-to-onset of 52 days (IQR 15–167), 89 days (IQR 29–199), 86 days (IQR 45–339), 84 days (IQR 28–343), 102 days (IQR 64–109) and 183 days (IQR 36–622), respectively.17 35
As the combination regimens of zanubrutinib are gaining clinical interest, we conducted a further search in the FAERS database for AEs associated with these combinations.36–39 According to our analysis, rituximab was the most frequently used agent in combination with zanubrutinib, with a total of 373 reports. Out of these cases, 166 were identified as the PS for either zanubrutinib or rituximab. AEs that occur at higher risk in patients treated with zanubrutinib plus rituximab include myelosuppression, pneumonia, leucopenia, decreased platelet count, abdominal pain, anaemia, pancytopenia, respiratory failure, pneumonitis and elevated blood lactate dehydrogenase. This is similar to the results of a phase II clinical study, which demonstrated that the combination of zanubrutinib and rituximab resulted in AEs with an incidence of≥10%, including leucopenia, neutropenia, anaemia, upper respiratory tract infection, elevated liver enzymes, haematuria, pneumonitis, decreased platelet count and purpura.37 In the analysis of zanubrutinib combined with chemotherapy, the highest risk was identified for cardiac toxicity, specifically the risk of cardiac arrest. This may be related to the significant cardiotoxicity of certain chemotherapeutic agents used in combination, such as cyclophosphamide, epirubicin and pirarubicin.
Our study had several limitations that were inherent to data mining research with the FAERS database. First of all, the FAERS is a self-reporting database that inevitably contains omissions, incomplete information, arbitrary reporting, misreporting and misinterpreted relationships, which can lead to potentially biased results in disproportionality analyses. Second, the analysis did not account for potential confounding factors, such as drug interactions and patient comorbidities, which could significantly influence the occurrence of AEs. Third, the disproportionality analysis only revealed a statistical correlation, rather than a clear causal association between the target drug and the specific AEs. Therefore, further causal evaluation is required, which may include reviewing drug labels, literature reports, expert opinions or conducting well-designed clinical trials. However, signals identified through big data analytics from postmarketing drug surveillance remain clinically significant in suggesting potential drug risk.
Conclusion
The safety profile of zanubrutinib was analysed in the real world, revealing a strong association with haematological toxicity, bleeding, infection and other AEs. These findings were consistent with the label and confirmed the reliability of this study. The analysis showed that zanubrutinib may be susceptible to AEs not listed on the label, such as abnormal hair texture, skin discolouration, hypernatraemia, pericardial effusion and hypersomnia. The time-of-event analysis showed that zanubrutinib-related AEs were characterised by an early failure profile, indicating that the risk of zanubrutinib-related AEs was higher in the early stage of treatment, with a decreasing risk over time. Furthermore, our study elucidates the increased risk of several AEs associated with the combination of zanubrutinib and rituximab, including myelosuppression, pneumonia, leucopenia, thrombocytopenia, abdominal pain, anaemia, pancytopenia and respiratory failure, compared with zanubrutinib monotherapy. Similarly, the combination of zanubrutinib with chemotherapy elevates the risk of nine AEs, such as cardiac arrest, elevated blood lactate dehydrogenase levels and pancytopenia. Our study provides important evidence for the clinical safety of zanubrutinib.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
Patient information in the FAERS database is anonymised. Therefore, ethical approval according to the Declaration of Helsinki is not required.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Contributors JW and XY conceived and designed the study. XZ, JL, JH and MZ performed the data extraction and data analyses. JW drafted the manuscript. PH and XY revised the manuscript. JW is the guarantor of the manuscript and accepts full responsibility for the work. All authors have read and approved the version of the manuscript submitted for publication. PH is the guarantor.
Funding This study was supported by the Research Program for Medicine and Health Science and Technology of Zhejiang Province (2022KY585, 2024KY761), Zhejiang Pharmaceutical Association Foundation Project (2020zyy22) and Zhejiang Medical Doctors Association Foundation Project (YS2022-3-009). Role of the funder: The funders had no role in the study, including collection, analysis, and interpretation of the data, writing of the manuscript, and the decision to submit the manuscript for publication.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.