Article Text
Abstract
Background Metronomic chemotherapy (‘less is more, regularly’) could be an alternative to the maximum tolerated dose (‘the more, the better’) in the chemotherapeutic cancer treatment of high-risk malignant solid extracranial tumours in children or young adults.
Objective To evaluate the efficacy of metronomic chemotherapy compared with placebo or stop treatment in paediatric patients with extracranial malignant solid tumours.
Methods We searched the databases MEDLINE and CENTRAL on 8 September 2023 and included randomised clinical trials (RCTs). Primary outcome was overall survival, and the main outcome measure was the HR.
Results We identified three RCTs with parallel assignment and intention-to-treat analyses of data from 775 people. The studies primarily reported on participants with rhabdomyosarcoma, neuroblastoma and osteosarcoma. The HR favoured the metronomic chemotherapy group (0.75 (95% CI 0.56 to 0.98)).
Conclusions The evidence base is compatible with a favourable effect of metronomic chemotherapy on children and young adults with high-risk extracranial malignant solid tumours, especially other than bone tumours, when compared with placebo or stop treatment. Statistical heterogeneity is low while clinical heterogeneity is substantial. Thus, the results must be interpreted with caution and applicability of the results is limited. Future RCTs could provide more data on individual tumour entities and subsequently add information on tumour-specific responses.
PROSPERO registration number CRD42023457195.
- paediatric oncology
- meta-analysis
- randomized controlled trial
- clinical decision-making
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The present systematic review is based on a comprehensive search.
Three included studies are characterised by a considerable number of participants and signs of low statistical heterogeneity among them.
The essential HR of overall survival was deduced from Kaplan-Meier curves.
Blinding was difficult to assess concerning the treatment of serious diseases, and we judged an unclear risk when blinding was not reported or not applied.
Applicability is limited due to the substantial clinical heterogeneity.
Introduction
Description of the condition
In the present systematic review, diseases of interest are malignant extracranial solid neoplasms that occur during childhood.1 This heterogeneous group of cancers represents approximately 40% of all paediatric malignancies in infants and young children.2 In children 0 to 14 years of age, the most common solid tumours are soft tissue sarcoma (7%), neuroblastoma (6%), nephroblastoma (5%) and malignant bone tumours (4%).2 Common presenting signs and symptoms of paediatric solid tumours are for example, abdominal mass, constipation, shortness of breath, back pain, bone pain, fever and arterial hypertension.2 Peripheral nervous cell tumours (such as neuroblastoma), bone sarcomas (such as osteosarcomas and Ewing sarcomas) and soft tissue sarcomas (such as rhabdomyosarcoma) separately affect about 1 child in 6000 to 7200 children under 18 years of age.3 More and more complex and intense treatment protocols have been established, and long-term survival significantly improved in recent decades.4 Nevertheless, survival after malignant extracranial solid tumour relapse is still poor. For instance, the reported 5 year overall survival rate after relapse from high-risk neuroblastoma is about 20% despite intense relapse treatments.5 New treatment strategies are urgently needed.6
Description of the intervention
Chemotherapy for paediatric extracranial high-risk tumours is usually based on the concept of maximum tolerated dose (MTD, 'the more, the better').7 High-dose chemotherapy aims to kill tumour cells but at the same time also threatens for example, bone marrow and organ function often leading to severe adverse events. Therefore, treatment-free intervals are needed to allow cell recovery. In contrast, metronomic chemotherapy (MC) is defined as the frequent administration of chemotherapeutic drugs at doses significantly below the MTD with no prolonged drugfree breaks (‘less is more, regularly’).8 As a multi-target treatment, MC is thought to affect the tumour microenvironment and the cancer cell. Effects on tumour angiogenesis and anticancer immunity have been shown.9 Additionally, MC is often combined with nonchemotherapeutic drugs that is, in the context of drug repurposing.10 The feasibility and the low toxicity profile in the heavily pretreated patients encouraged physicians to perform more clinical trials with metronomic treatment for solid tumours over the last years.
Objective
This systematic review aimed to evaluate the efficacy of MCcompared with placebo or stop treatment in paediatric patients with extracranial malignant solid tumours.
Methods
While preparing this systematic review, we endorsed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, adhere to its principles and followed its checklist.11 Patients and the public were not involved in this systematic review.
Search strategy
We performed an unrestricted electronic literature database search in MEDLINE via PubMed (US National Library of Medicine) on 8 September 2023. Due to automated term mapping, use of an asterisk (*) as a wild card for truncation, different spelling or use of synonyms was not necessary. We conducted an additional electronic literature database search in the Cochrane Central Register of Controlled Trials (CENTRAL) of The Cochrane Library via Wiley based on the PubMed search strategy. MeSH terms were combined with text terms to ensure a comprehensive and up-to-date search. We searched the online trial registries ClinicalTrials.gov (CT.gov, https://clinicaltrials.gov/, US National Library of Medicine) and International Clinical Trials Registry Platform (ICTRP, https://trialsearch.who.int, WHO) on 18 August 2023. The original and updated search strategies are shown in online supplemental table S1. We extended the search by using Google, reference lists of recent publications and PubMed tools including similar articles and clinical queries.
Supplemental material
Study selection
Inclusion criteria. We considered randomised controlled trials (RCTs) with parallel assignment of paediatric patients with extracranial malignant solid tumours. The test intervention was MC which generally followed standard treatment. MC was defined as the frequent administration of chemotherapeutic drugs at doses significantly below the MTD with no prolonged drugfree breaks.8 The control intervention was placebo or stop treatment. Exclusion criteria. We did not consider publications as follows: article abstracts, meeting abstracts, ongoing studies, trial registries, comments, letters, narrative reviews, systematic reviews duplicate publications.
Outcomes
Primary beneficial outcome measure was overall survival. Primary adverse outcome was treatment-related mortality. Secondary outcomes were progression-free survival, disease-free survival, event-free survival, toxicity grade 3 to 4 and health-related quality of life.
Data collection and analysis
FP imported the bibliographic data into Excel (Microsoft) and EndNote X9 (Clarivate), and FP and MH selected relevant studies in a two-step screening process. In the first step, selecting potentially relevant references was based on title and/or abstract. In the second step, including relevant studies was based on full text. Reasons were provided for excluding the rest of formerly potentially relevant articles. FP and MH also independently extracted information on study design, participants and outcomes into Word (Microsoft). Differences in opinions were resolved by discussion, and the assistance of a third author was not necessary. The main extracted data fields include the study characteristics (registry ID, design, sponsor, participating hospitals and enrolment), the participants (disease, age, gender, tumour histology, performance status, pathology, primary tumour invasiveness, regional lymph node involvement, tumour size and surgery), the interventions (pretreatment before and after randomisation and MC after randomisation), the comparators after randomisation, the treatment duration, the follow-up and the outcomes (overall survival, disease-free survival, progression free survival, event-free survival, health-related quality of life, death, treatment-related mortality, neutropenia grade 3 to 4, neurology adverse events grade 3 to 4 and nephrotoxicity grade 3 to 4).
Concerning time-to-event data, such as overall survival, we re-enacted the survival functions by deducing survival data from the survival curves depicted in the corresponding article. We used the Excel tool provided by Tierney et al12 to estimate the HR and the corresponding log(HR) by using the ‘data from curve with numbers at risk given’, which is based on the method by Parmar et al.13 If the numbers at risk were not given, we used the ‘data from curve read where wished and assuming constant censoring’. We made sure that the constructed graph based on input data was like the published graph. Data were analysed using the Cochrane Review Manager 5 (The Cochrane Collaboration): statistical method: inverse variance; analysis model: random effects and effect measure: HR. The procedure can be detailed as follows: first, the Kaplan-Meier plot is enlarged and printed. In general, time intervals are plotted on the x-axis and the probability of overall survival is plotted on the y-axis. The values of the time points are usually given in months such as 0, 12, 24 etc. The values of the probabilities are usually given as per cent such as 100% at time point 0. Second, a perpendicular is drawn between a specific time point of the x-axis and the corresponding point of the curve and the distance is measured. This is exerted twice, for the test group and for the control group. Third, the values of distance are converted in values of probability. The time point at 0 and the corresponding probability of 100% or 1.0 serve as a reference for the conversion. Fourth, the time points, the corresponding probability and the number of participants at risk are typed into the downloaded spreadsheet. It is important to make certain that the re-enacted curve mirrors the original curve perfectly. Fifth, the application calculates the HR and the log(HR). These figures are then transferred to the Cochrane Review Manager analysis tool.
Concerning dichotomous outcomes (eg, adverse events), we extracted the number of patients in each treatment arm and the number of patients who experienced the outcome of interest. Data were analysed using the Cochrane Review Manager 5 (The Cochrane Collaboration): statistical method: Mantel-Haenszel; analysis model: random effects and effect measure: risk ratio. We did not identify continuous data.
Subgroups and heterogeneity
We assessed clinical reasons for heterogeneity and estimated the percentage heterogeneity between trials that cannot be ascribed to sampling variation using the index of heterogeneity (I2 statistic).14 An I2 statistic equal to or greater than 50% was regarded as considerable heterogeneity. The studies did not separately report on bone sarcomas versus other than bone sarcomas, thus, it was not possible to conduct a sensitivity analysis in this regard. We conducted a subgroup analysis of studies on solid tumours other than bone sarcomas.
Assessment of risk of bias in included studies
Two authors independently appraised the risk of bias of the included studies. Differences in opinions were resolved by discussion and the assistance of a third author was not necessary. We used the items listed within Cochrane’s tool for assessing risk of bias.15 We assessed selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias. The criteria for judging an unclear, low or high risk of bias with respect to seven items are shown in online supplemental table S2.
Patient and public involvement
Patients and the public were not involved. There were no study participants since the study was based only on published study data.
Results
Search results
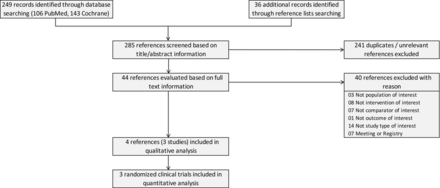
Figure 1 shows the literature retrieval and reference flow. We retrieved 285 references from electronic databases and additional sources such as references lists. We excluded 241 references based on title/abstract information. We excluded another 40 references with reasons based on full-text evaluation. We included four references16–19 which include the original study data of three RCTs16–18 and a follow-up report on health-related quality of life.19 During the 2022 Annual Meeting of the American Society of Clinical Oncology (ASCO), a poster referred to a study which appears to fulfil the inclusion criteria.20 Unfortunately, the study data relevant for the inclusion in this systematic review will not be available soon. Online supplemental table S3 lists the primary references for the three included studies and 10 associated references. Online supplemental table S4 lists the exclusion reasons for 40 references including the 10 references related to the included studies.
Literature search and flow.
Baseline data
Table 1 provides an overview of the main characteristics of the study design. Briefly, the three RCTs were published between 2017 and 2019. The screening of 1215 people resulted in enrolment and analysis of data of 775 participants from 16 countries (South America, Europe and Asia). All studies applied an intention-to-treat analysis and were sponsored by public institutions.
Inclusion criteria and study characteristics according to the included RCTs
Table 2 provides an overview of the main characteristics of the participants. Most participants were children or adolescents with slightly more males than females. The authors reported a variety of solid tumours mainly rhabdomyosarcoma, osteosarcoma and neuroblastoma. Patients of all three studies had previous treatments and were subjected to a high risk of relapse. Patients with cranial tumours were not included.
Online supplemental table S5 provides an overview of the main characteristics of the treatment. Patients in the test group received continuous low-dose chemotherapy in all three studies. Patients in the control group received placebo17 or stop treatment.16 18 Bisogno et al16 and Pramanik et al17 started the comparison at the time of the randomisation. Senerchia et al18 continued the pretreatment scheme for 18 weeks in both treatment groups after randomisation before starting the actual comparison.
Characteristics of study participants
Outcomes
Table 3 shows the extracted outcome data and adds the results of a re-enactment of overall survival data across all three included studies.
Type and results of reported outcomes
Primary outcomes
Figure 2 shows that the pooled estimate of overall survival favours MC when compared with placebo or stop treatment of malignant rhabdomyosarcoma, osteosarcoma and neuroblastoma: HR 0.75 (95% CI 0.56 to 0.98, p=0.04 and I2=6%). The results remained constant with a fixed effects model (data not shown). The funnel plot is compatible with a low publication bias and a sufficient number of included studies (online supplemental figure 1). We conducted a subgroup analysis to focus on tumours other than bone sarcomas and included the data from the study by Bisogno et al 16 and data of a separate analysis on tumours other than bone sarcoma from the study by Pramanik et al.17 Online supplemental figure 2 shows that the pooled estimate of overall survival favours MC when compared with placebo or stop treatment of malignant solid tumours other than bone sarcomas: HR 0.56 (95% CI 0.38 to 0.84, p=0.005 and I2=0%). Bisogno et al16 did not detect regimen-related deaths. Pramanik et al17 reported that there were no toxic deaths in both groups. Senerchia et al18 reported a regimen-related toxicity of 2% in both groups.
Supplemental material
Forest plot of metronomic chemotherapy versus placebo or stop treatment, outcome overall survival. I2, index of heterogeneity (the smaller the value the lesser the heterogeneity); IV, inverse variance (statistical method); P, probability; Random, random effects (analysis model).
Assessment of risk of bias in the included studies
Online supplemental table S6 lists the reasons for judging the risk of bias of the three included studies. Most items resulted in low or unclear risk of bias. A summary of the results of the risk of bias assessment is provided in figure 2 and online supplemental figure S2.
Discussion
Summary of main results
The findings from three RCTs suggest that MC for children and young adults with extracranial malignant solid tumours could improve overall survival when compared with placebo or stop treatment. One included study investigated the health-related quality of life and found no significant difference between groups. We did not identify any previously published meta-analysis.
Overall completeness and applicability of evidence
We believe that we have identified the relevant randomised trials studies sufficiently through searching CENTRAL, MEDLINE and ClinicalTrials.gov. CENTRAL is the result of actively screening for information on RCTs in various literature databases.21 According to Egger et al quote, “Investigators should consider the type of literature search and the degree of comprehensiveness that are appropriate for the review in question.”22 According to Peinemann et al, of 11 published RCTs on negative pressure wound therapy, all 11 were obtained from CENTRAL, 10 from MEDLINE, 9 from Embase and 3 from CINAHL.23 According to AMSTAR 2 quote, “at least two bibliographic databases should be searched.”24 All three included studies were published between 2017 and 2019, and we presume that the principal treatment procedures may not deviate considerably from the current practice. The studies were conducted by academic institutions, and a financial conflict of interest was not obvious. Senerchia et al18 reported a noteworthy number of dropouts but explained the reasons. We did not consider results on two biomarkers including those reported by Pramanik et al.25 The studies provided information required by the Consolidated Standards of Reporting Trials statement.26 All included studies have an explanatory attitude. In general, this means enrolment of highly selected participants in an ideal setting in contrast to an everyday medical practice.27 In addition, clinical heterogeneity among the included studies is substantial. Thus, the results have to be interpreted with caution and the applicability of the results is certainly limited.
Subgroups and heterogeneity
We assume significant clinical heterogeneity since various tumours were included within or across studies. The type of and the response to pretreatment varied among studies and included complete remission, refractory/progressive status and complete resection of the primary tumour. The Kaplan-Meier intervals ranged from 15 to 132 months. The drugs and their application (oral or intravenous) used for MC varied among studies. This obvious clinical heterogeneity may have a considerable impact on the applicability of the findings. MC is an umbrella term and may be applied with various active substances and doses. An identical MC plan may have different effects depending on the type of tumour.28 A subgroup analysis of other than bone sarcoma suggested that a possible favourable effect of MC may be primarily associated with rhabdomyosarcoma but not with bone sarcomas. We believe that populations and interventions were similar enough to be combined meaningfully in a meta-analysis. We included only paediatric patients and only interventions clearly defined as metronomic treatments. The I2 statistic of 6% is in line with the interpretation that there is not an important level of inconsistency. The random effects method and the fixed effect method gave identical results supporting a low heterogeneity among the studies. We chose the random effects model presumably giving a more conservative estimate of effect. The number of three included studies appears appropriate, especially in view of the inclusion of a total number of data from 775 participants. According to Ryan quote, “Two studies is a sufficient number to perform a meta-analysis, provided that those two studies can be meaningfully pooled and provided their results are sufficiently similar.”29
Potential biases in the review process
This systematic review applied a literature search with a degree of comprehensiveness appropriate to finding all available truly randomised studies. We chose overall survival as the primary outcome because it is the most reliable, patient-centred, sound and hard outcome in cancer studies. Grey et al listed examples of hard outcomes by clinical specialty.30 All three studies reported the Kaplan-Meier curves on overall survival but not all reported the HR and the corresponding log(HR), of which both values are necessary to pool the data. According to Higgins et al31 quote, “Conducting a meta-analysis using summary information from published papers or trial reports is often problematic as the most appropriate summary statistics often are not presented.” These statistics can be extracted from survival curves, and we deduced the essential data from the published Kaplan-Meier plots. Though the statistical heterogeneity was low, we used the random effects model as a precautional measure to calculate a conservative estimate. Nevertheless, clinical heterogeneity is obviously substantial. Therefore, the results have to be interpreted with caution and applicability of the study data to every day practice is certainly limited or possibly not warranted. Blinding is a challenge, or it is not possible when treating children with life-threatening diseases. Therefore, we judged an unclear risk when blinding was not reported or not applied.
Agreements and disagreements with other studies or reviews
We did not find a systematic review evaluating MC in children, and it does not seem meaningful to compare child cancer such as rhabdomyosarcoma and osteosarcoma with adult cancer such as breast cancer and colorectal cancer. This review substantially updates and improves the previous work in this area. The findings of this review generally agree with the findings in a recent summary review.28 32 One systematic review by Chen et alevaluated RCTs on metastatic colorectal cancer.33 The authors did not perform a meta-analysis due to substantial heterogeneity. With respect to overall survival, in all four included RCTs there was no significant difference between treatment arms. With respect to progression-free survival, there was no significant difference between treatment arms in two RCTs, and two RCTs reported in favour of MC. We do not agree with the results of the risk of bias assessment, and search strategies were not reported. Several other review type articles labelled ‘systematic reviews’ did not match the requirements defined by the PRISMA statement11 and AMSTAR 2.24 According to Sataloff et al quote, “Authors often submit articles that include the term systematic in the title without realizing that that term requires strict adherence to specific criteria.”34
Outlook
We believe that further RCTs are necessary to clarify the role of MC in the treatment of malignant solid tumours. Since different tumour entities or characteristics may react differently to this treatment, RCTs on specific neoplasms could provide important additional results. Health-related quality of life should be considered in studies on MC since this outcome is also critical for medical decision-making.
Conclusions
The evidence base is compatible with a favourable effect of MC on children and young adults with extracranial malignant solid tumours, especially other than bone tumours, when compared with placebo or stop treatment. Statistical heterogeneity is low while clinical heterogeneity is substantial. Thus, the results must be interpreted with caution and applicability of the results is limited. Future RCTs could provide more data on individual tumour entities and subsequently add information on tumour-specific responses.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Conceptualisation: FP and MH; resources: FP and MH; methodology: FP; writing – original draft: FP; writing – review and editing: MH; guarantor: FP.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.