Article Text
Abstract
Objective This study aimed to use systematic review and meta-analysis to establish the influence of antifungal therapy on pulmonary Candida colonisation of patients with mechanical ventilation (MV).
Design Systematic review and meta-analysis.
Data sources An extensive search was undertaken on publications from inception to 25 July 2023, through PubMed, Web of Science, Medline, Embase, China National Knowledge Infrastructure, Wanfang Data and VIP Databases.
Eligibility criteria for selecting studies Randomised trials, cohort studies and case-control studies comparing the efficacy of antifungal treatment in immunocompetent patients with pulmonary Candida colonisation after invasive ventilation.
Data extraction and synthesis Two reviewers independently extracted the data and assessed the quality of studies. Dichotomous outcomes were expressed as ORs with 95% CIs. Continuous outcomes were expressed as standardised mean differences (SMD) with 95% CIs.
Primary and secondary outcome measures The primary outcomes included intensive care unit (ICU), hospital, 28-day, and 90-day mortality. The secondary outcomes included ICU length of stay, MV duration and ventilator-associated pneumonia (VAP).
Results Nine high-quality studies were included. According to the data collected from these nine studies, there is no significant evidence showing a difference between the therapy group treated with antifungal drugs and the control group without antifungal drugs in clinical outcomes, including ICU mortality (OR: 1.37; 95% CI 0.84 to 2.22), hospital mortality (OR: 1.17; 95% CI 0.57 to 2.38), 28-day mortality (OR: 0.71; 95% CI 0.45 to 1.14), 90-day mortality (OR: 0.76; 95% CI 0.35 to 1.63), ICU length of stay (SMD: −0.15; 95% CI −0.88 to 0.59), MV duration (SMD: 0.11; 95% CI −0.88 to 1.10) and VAP (OR: 1.54; 95% CI 0.56 to 4.20). Subgroup analysis of different treatment types indicates that the combined effect size is stable and unaffected by different treatment types including inhalation (OR: 2.32; 95% CI 0.30 to 18.09) and intravenous (OR: 0.65; 95% CI 0.13 to 3.34).
Conclusion The application of antifungal treatment did not improve clinical outcomes in patients with MV. We do not suggest initiating antifungal treatment in patients with Candida pulmonary colonisation after invasive ventilation.
Trial registration number International Prospective Register of Systematic Reviews, CRD42020161138.
- Meta-Analysis
- Adult intensive & critical care
- Pulmonary Disease
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This study included a number of outcome indicators to assess the effectiveness of antifungal treatment, with subgroup analyses performed based on the type of treatment administered.
Most studies included are retrospective studies, raising potential concerns regarding their external validity.
Some studies described the precise style, name, dose and administration method of antifungal drugs, while others did not.
This study is limited by the number of studies included, especially when it comes to single outcomes such as intensive care unit mortality.
Most studies included were cohort studies instead of random control trials, which provide stronger evidence.
Introduction
Candida frequently exists in the normal oral cavity, upper respiratory tract, lower intestinal tract and vagina.1 In tracheal aspirates from patients with mechanical ventilation (MV), 30% have Candida isolation, and nearly 50% from patients suspected to have ventilator-associated pneumonia (VAP).2 3 When respiratory Candida colonisation is detected, it is difficult to differentiate between relatively harmless colonisation and invasive infection, and leads to a therapeutic dilemma.4 Although few studies reported limited attributable intensive care unit (ICU) mortality of VAP, the death rate related to VAP cannot be denied according to the majority of previous studies.5–8 The incidence of VAP frequently extends hospital stay, elevates the economic burden and raises the mortality rate.9 The presence of Candida in respiratory samples is defined as Candida colonisation instead of infection because the appearance of Candida pneumonia is rare.10–13 For several years, arguments have arisen about whether Candida colonisation negatively affects or simply indicates disease severity or immunosuppression in critically ill patients.13–15 We found that the outcomes of antifungal treatment for Candida colonisation in pulmonary tract among different studies were controversial, as some studies found that antifungal treatment was associated with lower mortality, some found that treatment made no difference in mortality, while others found that it prolonged MV duration, ICU length of stay and hospital length of stay.10 11 16 17 Therefore, we performed a systematic review and meta-analysis to establish the influence of antifungal treatment on pulmonary Candida colonisation.
Method
Information sources and search strategy
This study followed the Preferred Reporting Items for Systematic Review and Meta-Analysis criteria for systematic reviews and meta-analyses.18 An extensive search was undertaken on publications from inception to 25 July 2023, through PubMed, Web of Science, Medline, Embase, China National Knowledge Infrastructure, Wanfang Data and VIP Databases. The searching syntax included the following Medical Subject Headings and text words, which are varied individually according to different databases: VAP, Candida. We used the following search strategies in all of the databases above: (ventilator-associated pneumonia OR VAP OR pneumonia, ventilator-associated OR ventilator-associated pneumonia OR ventilator-acquired pneumonia OR mechanical ventilation) AND (Candida OR Candida spp OR Candida colonization OR Candida airway colonization). There were no language restrictions on studies searching. The listed references of relevant studies were also evaluated to enlarge the search scope and ensure a complete search. Full search strategy of PubMed database is provided in the online supplemental material. Selection process of the article was performed by two researchers (LL and SS) independently. The titles and abstracts of the entries identified in the search were screened. Full-text version of all articles that potentially met the eligibility criteria was reviewed to make a decision. Disagreements, if any, were resolved through discussion with a third researcher (H-BX).
Supplemental material
Inclusion criteria
Randomised trials, cohort studies and case-control studies were included while selecting. In the included studies, the patients were adults (≥18 years old) who were diagnosed with pulmonary Candida colonisation after invasive ventilation. Pulmonary Candida colonisation was defined as the presence of Candida in bronchoalveolar lavage samples, endotracheal aspiration samples, protected brush specimens or any positive airway secretion specimens.
Exclusion criteria
We excluded case reports and studies on pregnant, immunocompromised patients or those who received antifungal treatment for reasons other than Candida colonisation. Patients with candidemia or invasive candidiasis were also excluded, and the diagnostic criteria were positive results of direct detection, in which blood or tissue specimens were cultured, or indirect detection, in which surrogate markers and PCR assays were used.19
Data collection
We screened titles and abstracts, reviewed the full text and extracted data using an Excel sheet (Microsoft Corporation). We primarily collected the characteristics of title, author, journal, year, type, participants, inclusion or exclusion criteria, interventions and clinical outcomes. Clinical outcomes included 28-day mortality, 90-day mortality, ICU mortality, hospital mortality, ICU length of stay, MV duration and VAP. Disagreements were resolved by discussion with a third reviewer if necessary.
Quality assessment
We used the Cochrane risk assessment tool to measure the risk of randomised-controlled trials, and the Newcastle–Ottawa scale to measure the quality of cohort and case-control studies.20 Studies that scored at least six points were regarded as high quality and included in the analysis.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Statistical analysis
Statistical analysis was performed by Stata software (V.16.0). Dichotomous outcomes, such as VAP, ICU mortality, hospital mortality, 28-day mortality and 90-day mortality, were expressed as ORs with 95% CIs. Continuous outcomes (ICU length of stay and MV duration) were expressed as standardised mean differences (SMD) with 95% CIs. We analysed the data using the inverse-variance method with the fixed-effect model if there was no obvious heterogeneity (p>0.1), or else random-effect model if the heterogeneity was significant (p<0.1). Moreover, heterogeneity was quantified using the I2 test. The interpretation of I2 was guided by the Cochrane Handbook for Systematic Reviews of Interventions (V.5.2.0, updated June 2017). Sensitivity analyses were conducted by excluding one study at a time from the analysis to assess the stability of the results. Subgroup analyses were performed on the effect of antifungal therapy. Publication bias was not assessed since there were fewer than 10 studies in this meta-analysis.
Results
Study selection
In total, 2947 records were identified. With 1246 duplicates removed and 1670 irrelevant records excluded, we assessed 31 studies for eligibility, of which 9 were included. 22 studies were excluded because of the lack of antifungal treatment, lack of supporting experimental data, lack of detection of pulmonary secretion and occurrence in a special population (figure 1). All patients received MV, had at least one positive culture of Candida in the pulmonary tract and received different antifungal treatments.
Flowchart of studies identified, excluded and included.
Study characteristics and quality assessment
Of the nine articles included in the study, seven were retrospective cohort studies, one case-control study and one randomised clinical trial. Two studies were conducted in North America, five in Europe and two in Asia, giving the studies a wide geographical coverage. Other characteristics of the included studies are summarised in online supplemental table 1, including years of accuracy, location, study design, population, number of patients, outcomes, Candida colonisation and antifungal treatment. The columns of the quality assessment list and their corresponding points are listed in online supplemental table 2. All included studies were assessed to be of high quality.
Effect of antifungal therapy on pulmonary Candida colonisation patients’ mortality
Three studies, including Ioannou,2 Ong21 and Nseir,22 reported ICU mortality. We found no significant difference between the therapy and control groups (OR: 1.37; 95% CI 0.84 to 2.22). Six studies, including Du,4 Ioannou, Zhang,23 Griffin,24 Lindau25 and Albert,26 reported hospital mortality. The pooled results showed no significant difference between the therapy and control groups (OR: 1.17; 95% CI 0.57 to 2.38). Four studies, including Du, Zhang, van der Geest27 and Albert, reported 28-day mortality. The results showed no significant difference between the therapy and control groups (OR: 0.71; 95% CI 0.45 to 1.14). Three studies, Du, van der Geest and Albert, reported 90-day mortality. The results showed no significant difference between the therapy and control groups (OR: 0.76; 95% CI 0.35 to 1.63). No indicator showed statistical significance with respect to mortality (figure 2).
(A) Forest plot for intensive care unit mortality. (B) Forest plot for hospital mortality. (C) Forest plot for 28-day mortality. (D) Forest plot for 90-day mortality.
Effect of antifungal therapy on pulmonary Candida colonisation patients’ ICU length of stay
Four studies, including Zhang, Griffin, Ong and Nseir, reported ICU length of stay. The results indicated non-significant differences for ICU length of stay among patients receiving antifungal treatment (SMD: −0.15; 95% CI −0.88 to 0.59), as shown in figure 3.
Forest plot for intensive care unit length of stay. SMD, standardised mean difference.
Effect of antifungal therapy on pulmonary Candida colonisation patients’ MV duration
Three studies, including Zhang, Griffin and Nseir, reported the duration of MV. We did not identify a significant difference between the therapy and control groups regarding MV duration (SMD: 0.11; 95% CI −0.88 to 1.10), as shown in figure 4.
Forest plot for MV duration. MV, mechanical ventilation; SMD, standardised mean difference.
Effect of antifungal therapy on VAP
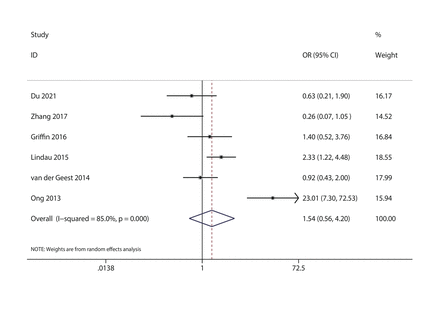
Six studies, including Du, Zhang, Griffin, Lindau, van der Geest and Ong, reported VAP. No significant difference was found between therapy and control groups (OR: 1.54; 95% CI 0.56 to 4.20), as shown in figure 5.
Forest plot for ventilator-associated pneumonia.
Subgroup analysis
We conducted a subgroup analysis based on the effect of antifungal therapy on VAP of the included studies. The result of the subgroup analysis indicates that the combined effect size is stable and unaffected by different treatment types, as shown in online supplemental figure 1.
Sensitivity analysis and publication bias
A sensitivity analysis was performed by sequentially excluding one study at a time. This exclusion did not significantly impact the results, with the pooled OR ranging from 1.00 (0.51–1.96) to 2.07 (0.74–5.78) (online supplemental figure 2). Because only nine related studies were included in this report, approaches for detecting publication bias would have exhibited limited efficacy. Consequently, the evaluation of publication bias was not conducted.
Discussion
In this study, we observed that antifungal therapy administered to mechanically ventilated patients with Candida colonisation did not show a significant impact on patient outcomes, which were measured by indicators, including mortality, hospital length of stay, ICU length of stay, MV duration and incidence of VAP, and the result is stable when taking different treatment types into consideration. A study by Du declared that antifungal treatment was associated with a reduced risk of VAP, while an autopsy study involving 232 samples showed that, although Candida is a common pathogen, the incidence of Candida pneumonia in ICU patients is extremely low.4 11 Inconsistent with the results of the previous studies, a meta-analysis found that Candida pulmonary colonisation probably had poorer clinical outcomes, owing to longer MV duration, higher 28-day mortality, higher ICU mortality and longer ICU length of stay.28 Furthermore, a study by Ioannou found that about half of the patients with Candida spp. isolation from their respiratory secretions were treated with antifungals. Considering the factor that patients under more critical condition could be treated with antifungals more often, a multivariate regression analysis was conducted identifying antifungal use as an independent factor associated with total hospital mortality.2 Without listing the specific articles for verification, the guideline strongly recommends that Candida colonisation rarely requires treatment with antifungal therapy.29 Our study may help to provide more information on this problem: whether Candida pulmonary colonisation simply symbolises the severity of diseases or actually has an influence on outcomes.30 31
Candida colonisation in the respiratory tract is related to higher inflammation and may accelerate the disease process.32 33 A few studies used inhalation of antifungal drugs as therapy, which did not show a significant influence on patients’ outcomes. This may lead to direct inhibition or damage of the alveolar-capillary membrane, resulting in an influx of surfactant-inactivating plasma proteins.21 27 After Candida colonisation, current increases in antifungal drug resistance in Candida spp. and clinical treatment failures are of concern.34 Previous history of antifungal prescription influences Candida species distribution and susceptibility profile to antifungal agents.35 Inadequate dose and treatment failure may contribute to high mortality.36
Considering the various conditions, we thought that various factors cause poor clinical outcomes: antifungal treatment may have more harm than benefit in clinically ill patients. Different antifungal drugs can have varying sensitivities or resistances as well as potential toxicity or adverse effects. Inadequate dosing of antifungal drugs may also be a contributing factor. Although the analysis of this study showed that antifungal treatment did not improve patients’ clinical outcomes, the sample size of included studies was limited and the 95% CIs were wide. Further studies should be conducted to verify their influence. Considering retrospective and current studies can only provide hypotheses to support the existence of a correlation, a prospective randomised controlled trial might be a more appropriate solution to explore the effect of antifungal treatment on patients with respiratory Candida colonisation in combination with MV.
Based on the findings of this meta-analysis, the use of antifungal medication on mechanical-ventilated patients with respiratory Candida colonisation does not appear to improve patients’ clinical outcomes. No significant differences were observed in ICU mortality, hospital mortality, 28-day mortality, 90-day mortality, ICU length of stay, MV duration and VAP associated with different treatment regimens. Further analysis of subgroups based on different treatment types confirmed these conclusions. Therefore, the use of antifungal medication is not recommended for the decolonisation of mechanical-ventilated patients with respiratory Candida colonisation.
The strength of this study is that it included a number of outcome indicators to assess the effectiveness of antifungal treatment, with subgroup analyses performed based on the type of treatment administered. However, it encountered several limitations. Most studies included are retrospective studies, raising potential concerns regarding their external validity. Additionally, some studies described the precise style, name, dose and administration method of antifungal drugs, while others did not. Furthermore, this study is limited by the number of studies included, especially when it comes to single outcomes, such as ICU mortality. Moreover, most studies included were cohort studies instead of random control trials, which provide stronger evidence.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
LL and SS contributed equally.
Contributors H-BX and HY provided article ideas and completed a literature search. LL and SS contributed to the data analysis and manuscript writing. H-BX is the guarantor.
Funding This study was funded by Changsha Municipal Health Committee (No.KJ-B2023032).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.