Article Text
Abstract
Introduction During the last decade, extensions of therapeutic indications have been one of the most common methods to extend the lifecycle of a medical product in the post-authorisation phase and to increase the use and sales of medicines. The aim of this study was to gain understanding of the lifecycle of cancer medicines and especially the role and level of evidence extensions in comparison to first indications.
Materials and methods We identified all new outpatient cancer medicines approved by the European Medicines Agency between 2010 and 2020 and the extensions to their indications. We compared general study design characteristics from the European public assessment reports using critical appraisal tools and clinical added value assessments.
Results We identified altogether 55 new outpatient cancer medicines, 31 of which had one or more extension(s) of indication and 24 had no extension of indication. In total, there were 57 extensions. The most common extension of indication was a change in the treatment line (35%). Compared with first indications, the overall quality of studies supporting extensions was better in terms of study designs. The proportion of medicines providing CAV was higher in extensions compared with first indication of medicines with and without extensions.
Conclusions Based on different assessments and perspectives, we found that extensions of indications are a very common and important part of extending the lifecycle of outpatient cancer medicines in Europe. Our findings also suggest that the clinical value of cancer medicines increases with extensions.
- clinical trials
- health policy
- oncology
Data availability statement
All materials are publicly available. EPARS: https://www.ema.europa.eu/en/medicines. Clinical added value assessments: https://www.has-sante.fr/jcms/pprd_2986129/en/home. Data are available upon reasonable request and most of the data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
We analysed all European public assessment reports of new outpatient cancer medicines with or without extensions of indications during 2010–2020.
We used multiple perspectives in the assessment: the characteristics of the medicines and study designs, the quality of clinical studies by Joanna Briggs Institution Assessment tools and the assessment of clinical added value using Haute Autorité de Santé evaluations.
It is possible, that we missed some extensions of indications if they were approved after our data collection.
This study was descriptive in its nature and due to the low number of observations we were unable to detect any statistically significant differences between the medicines with or without extensions of indications.
Our study provides an integrated understanding of the role of extensions of indications from the European perspective.
Introduction
Cancer medicines have been one of the key medical innovations in last decade. In the current niche-buster pharmaceutical market, different methods are used to extend the lifecycle of medicines.1 2 Extensions of therapeutic indications are one of the most common methods to extend the lifecycle of a medical product in the post-authorisation phase and to increase the use and sales of medicines.3–6 In Europe, extensions allow the innovator company an additional period of data exclusivity and market protection lasting at least a year.7 8 Nowadays, extensions of indications have become more common than the acceptance of new active substances.9 10
Marketing authorisation (MA) holders aim to get new cancer medicines approved as soon as possible and expanding indications is common.11 A study on targeted multi-indication cancer medicines found that medicines are first accepted as monotherapies in rare diseases with less mature evidence often based on single-arm studies and surrogate endpoints.4 Extensions of indications are generally targeted to broader populations and based on more mature evidence. On the other hand, extension of indications may have minor clinical importance than the first approved indications.12 A recent US analysis also revealed the importance of extensions of indications for the so-called partial orphan medicines, thus medicines initially intended to treat both rare and common diseases and how they are turned into blockbuster medicines.13 However, many of the previous findings focusing on the role of extensions of the indications are based on the medicines approved in the USA.
Another major trend in cancer medicine market is the shift towards outpatient cancer care, driven by the desire to use inpatient care resources more rationally, improve cost-efficiency and patient experience and avoid hospitalisation.14 Although outpatient cancer care has become more important in recent decades, to our knowledge no previous study has focused on outpatient cancer medicines and their extensions of indications. Extensions of indications maybe even more important for outpatient medicines than for inpatient medicines, as their potential uptake is indication-based.15
Many publications have questioned the actual benefits of the new cancer medicines, as their impact and evidence on survival and quality of life is very limited.16–18 In order to better understand the value of outpatient cancer medicines and the role of extensions of indications, it is important to gain a more comprehensive understanding of first and later indications of cancer medicines and the quality of the research evidence supporting their approvals.
The quality of research can be assessed with different critical appraisal tools.19 One of the most common methods is the critical appraisal tools of the Joanna Briggs Institute (JBI),20 which include comprehensive checklists for different types of study settings.21 In addition to the quality of study designs, it is crucial to assess the clinical added value (CAV) of new medicines. CAV takes into account and compares the efficacy and safety of a medicine with existing treatments. One validated instrument for this kind of work is the French Haute Autorité de Santé (HAS), whose CAV assessments are publicly available.22
The aim of the study was to explore the role and the level of evidence of extensions of indications in the European cancer medicine approvals. More specific aims were (1) to describe and compare the new outpatient cancer medicines and their extensions, (2) to evaluate and compare the evidence at the MA acceptance phase between the following three groups: first indications for multi-indication medicines, extensions and medicines without extensions and (3) to analyse and compare the CAV between these three groups.
Materials and methods
Data collection
Our study focuses on new cancer medicines that received MA for the first time in 2010–2020 and possible extensions of indication by the end of 2022, in addition to which they are suitable for outpatient care by their administration route (online supplemental figure 1), that is, the active substances are targeted to tumour tissue based on Anatomical Therapeutic Chemical (ATC) codes L01, L02, L04AX02, L04AX04 and L04AX06.23 Data were collected from European Medicines Agencies (EMA’s) website and the European public assessment reports (EPARs).24 The latest data collection took place in June 2023. We categorised the types of extensions of cancer medicines into five categories (online supplemental table 1) based on a list by the European Commission.25 In addition to these categories, we added one more: multiple changes. We classified new cancer medicines into 10 groups by the target tissue of their first indication (table 1). We used level four ATC groups (chemical subgroups)26 to estimate the number of new mechanisms of action.
Supplemental material
Characteristics of new outpatient cancer medicines by cancer type according to the first approved indication of the medicine
Quality assessment using the Joanna Briggs Institute critical appraisal tools
The quality of the main studies from EPARs was assessed by using the JBI checklist for randomised controlled trials (RCT), checklist for quasi-experimental studies and checklist for systematic reviews.20 The JBI checklists were selected due to their comprehensibility and because separate checklists were available for different study settings.21 The checklists for RCT, quasi-experimental studies and systematic reviews contain 13, 9 and 11 questions, respectively. Each question can be assessed as yes, no, unclear or not applicable.
The quality assessments were conducted separately by two researchers (A-MR and TK). Any discrepancies were discussed until a consensus was reached. After all the assessments, the questions were divided into four categories by theme in order to summarise the different checklists and their results.
Clinical added value by the assessment of Haute Autorité de Santé
HAS is the independent French National Authority for Health that, among other things, assesses applications for reimbursement of new medicines. HAS will assess the actual clinical benefit (ACB) and decides whether to recommend a medicine for reimbursement. For this study, we used the publicly available HAS evaluations of CAV scored on a scale of no improvement, minor, moderate, substantial and major.27 We classified medicines with no ACB and no evaluation of the medicine or indication by the HAS under the no improvement category. It reflects the overall situation where a new medicine adds no clinical value. We collected assessments for the first indications and subsequent extensions of indications in June 2023. Another popular, validated instrument for the assessment of CAV is the European Society for Medical Oncology (ESMO) Magnitude of Clinical Benefit Scale (MCBS).28 However, at the time of our study, MCBS scales did not include the evaluation of medicines for haematological indications.29 Because HAS evaluations include also medicines for haematological cancer, we used HAS evaluations in this study.
Patient and public involvement
Patients and members of the public were not involved in the design and conduct of this study.
Results
Characteristics of medicines and extensions of indications
We identified altogether 55 new outpatient cancer medicines approved by EMA between 2010 and 2020 accounting for more than half (53%) of all new cancer medicines approved (online supplemental table 2). The most common indications of these medicines were the treatment of haematological cancers (24%, n=13), lung cancer (16%, n=9) and melanoma and basal cell carcinoma (15%, n=8) (table 1). More than half (56%, n=31) of all new cancer medicines had received at least one extension of indication. The remaining medicines (44%, n=24) had no extensions of indication. Most commonly, extensions (n=57) involved a new treatment line (35%, n=20), a new cancer type (30%, n=17) or a new combination therapy (18%, n=10). We found only three extensions of indications to new patient groups (5%) and all were lung cancer medicines. We found six extensions, classified as multiple changes (11%) in the following medicine groups: haematological cancers (n=3), gynaecological cancer (n=2) and lung cancer (n=1).
A majority (77%) of medicines approved for the treatment of haematological cancers were launched with a new mechanism of action (table 1), while one-third of medicines for lung, gynaecological and thyroid cancers, had a new mechanism of action. The medicine that was the first in a new ATC group often had the highest number of extensions. In our data, the first active substance in the ATC group had the highest number of extensions in 7 out of 21 different ATC groups (33%) during the follow-up period. Furthermore, most extensions came from other than the first active substance in 4 (19%) ATC groups, and 7 (33%) ATC groups had only one active substance. In the remaining groups (14%), all medicines had the same number of extensions. Medicine-specific characteristics are presented in online supplemental table 2.
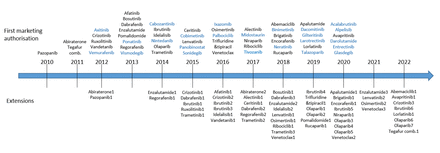
Of the 31 medicines with extensions of indications, 19 had only one and 12 had two or more extensions (figure 1). The maximum number of extensions was seven (for olaparib). The timeline in figure 1 shows when the new active substances received their first MA and when their extensions of indication were approved. On average, the first extension of indication was granted 2 years and 7 months after the first MA (minimum 7 months; maximum 10 years and 10 months; median 2 years and 1 month). The average time between the first and second extension of indication was 2 years and subsequent extensions were granted in less than 2 years, on average.
Timeline of the approved medicines with and without extensions of indications. Medicines without extensions are indicated in blue.
Study designs and marketing authorisations
In total, 124 main studies were identified and evaluated. In 13 cases, there were two main studies. Most of the main studies supporting the first MA or extensions of indications were phase III studies with randomised controlled study design (80%, figure 2). Phase I–II non-controlled single-arm trials were a more common study design for the first indication of medicines with extensions (32%) than for other groups (12% and 17%).
Study designs and main outcome variables of the main studies, comparison of the first indication of the medicines with extensions (n=31), the extensions of the indications (n=57) and the medicines without extensions of indications (n=24). *Controlled study design includes both active-controlled and placebo-controlled studies. For two medicines, their extensions were based on the same active-controlled studies. *In addition to designs presented, one medicine’s (tegafur combination) extension is based on a meta-analysis. MA, marketing authorisation.
Medicines with extensions were more likely to have a conditional MA application than medicines without extensions (26% and 13%, respectively). Most (86%) of the main studies used surrogate endpoints (such as progression-free survival or overall response rate as the main outcome variable (figure 2). Overall survival was rarely used as the main endpoint and was more common in the studies on medicines without extensions (21%) than in the other groups (12% and 13%). In addition, ORR was most frequently used as a key outcome variable in the studies (42%) of the first indication of the medicines with extensions while its use was less frequent in the other groups (16% and 17%).
The majority of all new cancer medicines (85%, n=47) were indicated for the treatment of advanced or metastatic disease at the time they received their first MA. Treatment of early-stage condition was more common for extensions of indications than for other groups.
Evaluation of the quality of evidence
Based on the JBI assessment, the overall quality of the main studies on extensions and medicines without extensions was better than that of the first indications of medicines with extensions (good and unclear in figure 3). This is explained by the larger proportion of phase III RCTs in the study designs. When only the studies with good assessments of quality are considered, medicines without extensions received the best rating in three out of four categories.
Quality of main studies assessed against Joanna Briggs Institute-criteria, comparison of the first indication of the medicines with extensions (n=31), the extensions of the indications (n=57) and the medicines without extensions of indications (n=24).
In many studies, details of the randomisation and double-blinding were missing. Double-blinding was well-described in up to one-third of the studies. However, almost half of all main studies of all medicines did not have a double-blind design (figure 3). Medicine-specific assessments are presented in online supplemental tables 2 and 3.
In the assessment of the similarity between the compared groups, less than half of the studies were evaluated to fill the criteria of good quality. The most common reasons for poor quality of studies were crossover between groups, different follow-up times in different populations and, in some cases, different previous treatments in the compared groups.
Clinical added value
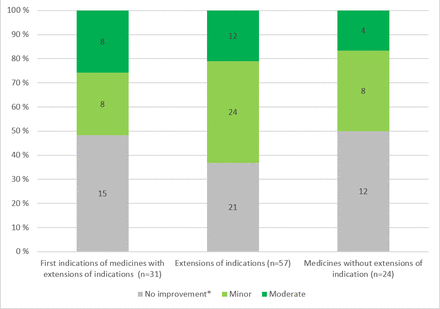
Overall, extensions of indications had the highest scores in the CAV assessment (minor and moderate CAV in 63%; figure 4). In the other two groups, almost the same proportion of medicines had some CAV (52% vs 50%). Moderate was the highest CAV estimate of the data set, and it should be noted that none of the indications provided substantial or major CAV. In terms of percentages, the highest moderate ratings were for the first indication for medicines with the extension of indication (26%). Moderate assessments focused particularly on products for the treatment of prostate cancer, haematological cancers and melanoma. Medicine-specific assessments are presented in online supplemental table 2.
Assessment of clinical added value by Haute Autorité de Santé. Comparison of the first indication of medicines with extensions of indication (n=31), extensions of the indications (n=57) and medicines without extensions of indications (n=24). *The category ‘no improvement’ included also medicines for which no assessment was available (n=9) or actual clinical benefit was insufficient (n=9).* Includes situations where the actual clinical benefit is insufficient or no assessment is available.
Discussion
Based on different assessments and perspectives, we found that extensions of indications are a very common and important part of extending the lifecycle of outpatient cancer medicines in Europe. Our findings also suggest that the clinical value of cancer medicines increases with extensions. In more detail, first, the most common category of extensions was a change in the treatment line, that is, a tendency to push the use of a cancer medicine to an earlier point in the treatment line and, thus, increase the number of potential users and extend the duration of treatment. Second, based on the characteristics of study design and JBI evaluation, extensions of indications are based on improved quality of evidence compared with first accepted indications. In addition, according to CAV assessments, extensions add more clinical value than the first indications. Looking at the different measures and perspectives, it appears that extensions of indication are of higher quality than the first indications of evaluated medicines.
Evidence supporting extensions of indications was of higher quality
Our study is in accordance with previous findings4 11 30 suggesting that new outpatient cancer medicines are brought to market with less comprehensive clinical evidence, which is to be improved in later indication extension studies. This is linked to, for example, the number of conditional MAs and phase I–II studies. It also seems that conditional MA is more common for medicines with extensions than for those without them. Furthermore, we also found that in studies of extensions of indications yielded a higher overall CAV than the studies of those medicines whose indications were subsequently extended and those medicines without extensions. This finding is slightly different from the findings of a study using ESMO MCBS,31 in which original indications were scored higher than extended indications.31 This can be explained by the different assessment scale used or by the fact that we included also haematological indications in our study.
Change in treatment line was the most common extension type
In our study, the most common type of extension was a change in the treatment line. This was seen, for example, in prostate cancer, where androgen receptor signalling inhibitors abiraterone, enzalutamide and apalutamide were first indicated to castration-resistant prostate cancer and later extended to earlier hormone-sensitive stages of the disease. This pattern was similar also in metastatic lung cancer and ALK (anaplastic lymphoma kinase) inhibitors (crizotinib, alectinib, ceritinib, brigatinib and lorlatinib), all of which were initially indicated as second-line or third-line treatment but received extensions to first-line treatment over time. This reflects the fact that cancer medicines often initially enter the later line and move to an earlier stage of treatment with extensions. The second most common type of extension was a new cancer type, which was particularly common for colorectal and gastric cancer medicines. These medicines (tegafur combination, trifluridine and tipiracil, regorafenib and avapritinib) are not targeted to specific signalling pathways (like androgen receptors in prostate cancer or EML4-ALK [echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase] translocations in lung cancer), which explains the rationale to investigate their potential in cancers of different origin. New combination therapies were particularly common in haematological indications. For other extension types, only a few medicines were included and for instance, the extension to new patients was only found in three lung cancer medicines.
Medicines with new mechanism of action had most extensions of indications
According to our data, it is common that the first-to-market products with a new mechanism of action have the highest number of extensions. To our knowledge, there are no previous findings on this. A previous North American cross-sectional study32 showed that only a minority of Food and Drug Administration-approved cancer medicines during 2009–2020 were based on a new mechanism of action. Our findings indicate that the first entrant can be characterised as a trendsetter, and subsequent medicines will, in most cases, have the same indication(s) as the first medicine. The importance of new mechanism of action and subsequent extensions should be studied more, also in different therapeutic areas.
Implications for patient care and policy
Looking at the research design and the quality of the evidence, it seems that a new mainstream of medicine approval has emerged over the last decade. For example, previous research33 suggests that the majority of new cancer medicines from 1995 to 2008 had only one indication. This is the opposite of the current situation with medicines with multiple extensions targeted to larger populations. The current drive is to provide new treatments to patients as quickly as possible. This trend can also have a negative impact on patient care and outcomes. On the other hand, for some medicines, lighter approval criteria are beneficial for the uptake of medicines and, therefore, for patients.34 At the beginning of 2025, the new Regulation on Joint Health Technology Assessment at the European Union level is applied.35 One important aspect to consider in the joint evaluation of the evidence is the possible extensions of indications and how they are addressed. The results of this study may increase of the overall understanding among authorities and decisions-makers of the role of extensions of indications, which can help in future medicine assessments. For instance, it is worth considering whether the extension of indication or the first indication becomes the main indication for a medicine, and what impact it has on the number of medicine users and the resulting costs.
Strengths and limitations
Although previous analyses4 11 have assessed the evidence related to extensions of indications, to our knowledge, our study includes more medicines than previous analyses, with a particular focus on European outpatient cancer medicines. Our study included also cancer medicines with haematological indications, accounting for almost a quarter of all new outpatient cancer medicines approved. The strength of this study is that it was based on publicly available documents from the EMA on all new cancer medicines suitable for outpatient use in Europe between 2010 and 2020 using multiple essential assessment methods. We also provide detailed, medicine level information in the online supplemental tables 2 and 3. However, our study is not without limitations. First, the median time to the first extension was 2 years and 1 month. Based on this, we believe that the follow-up period of our study (until spring 2023) is long enough to capture the majority of the potential extensions of the indications. However, it is possible that some of the products have extensions after the data collection period has ended. We used the JBI critical appraisal tools to assess methodological quality because of their comprehensibility21 and because JBI checklists exist for different types of study settings. In the assessment of CAV, we chose to use HAS assessments because they are performed for most medicines, including haematological indications. It is possible that the assessment tools we used have influenced our results. Finally, due to the low number of observations we were unable to detect any statistically significant differences between the observed medicine groups (first indications, extensions of indications and medicines without extensions). Overall, we consider the utilisation of the different kinds of assessments and perspectives give us a comprehensive understanding of the evolvement of the evidence during the lifecycle of the studied medicines and especially the important role of extensions of indications in extending the lifecycle of outpatient cancer medicines in Europe.
Data availability statement
All materials are publicly available. EPARS: https://www.ema.europa.eu/en/medicines. Clinical added value assessments: https://www.has-sante.fr/jcms/pprd_2986129/en/home. Data are available upon reasonable request and most of the data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
A-MR and TK are joint first authors.
X @Kurkota, @KatiSarnola, @hannakkoskin
A-MR and TK contributed equally.
Contributors Concept and design: TK, KS, HK. Acquisition, analysis or interpretation of data: All authors. Drafting of the manuscript: A-MR, TK. Critical revision of the manuscript for important intellectual content: All authors. Supervision: TK, KS, KK, HK. TK is responsible for the overall content of the manuscript (as guarantor).
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.