Article Text
Abstract
Introduction First-line oxygenation strategy in patients with acute hypoxaemic respiratory failure consists in standard oxygen or high-flow nasal oxygen therapy. Clinical practice guidelines suggest the use of high-flow nasal oxygen rather than standard oxygen. However, findings remain contradictory with a low level of certainty. We hypothesise that compared with standard oxygen, high-flow nasal oxygen may reduce mortality in patients with acute hypoxaemic respiratory failure.
Method and analysis The Standard Oxygen versus High-flow nasal Oxygen-trial is an investigator-initiated, multicentre, open-label, randomised controlled trial comparing high-flow nasal oxygen versus standard oxygen in patients admitted to an intensive care unit (ICU) for acute respiratory failure with moderate-to-severe hypoxaemia. 1110 patients will be randomly assigned to one of the two groups with a ratio of 1:1. The primary outcome is the number of patients who died 28 days after randomisation. Secondary outcomes include comfort, dyspnoea and oxygenation 1 hour after treatment initiation, the number of patients intubated at day 28, mortality in ICU, in hospital and until day 90, and complications during ICU stay.
Ethics and dissemination The study has been approved by the central Ethics Committee ‘Sud Méditerranée III’ (2020-07-05) and patients will be included after informed consent. The results will be submitted for publication in peer-reviewed journals.
Trial registration number NCT04468126.
- Adult intensive & critical care
- Pulmonary Disease
- Respiratory infections
- Respiratory Therapy
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is the largest study comparing high-flow nasal oxygen with standard oxygen in patients with acute hypoxaemic respiratory failure in view of assessing their impact on mortality and intubation.
The population of this study will include patients at high risk of intubation and mortality, that is, patients with moderate to severe hypoxaemia defined by the partial pressure of arterial oxygen to fractional inspired oxygen ratio equal to or below 200 mm Hg.
Intubation criteria will be predetermined in order to avoid delayed intubation. The same predetermined criteria of intubation will be defined in both groups, allowing comparison of time to intubation in each arm and its relationship with survival.
The treatment allocated to patients cannot be masked, due to the type of oxygen supports evaluated in the study. However, the coordinating centre and all the investigators will remain unaware of the study group outcomes until the data is locked.
Introduction
Background and rationale
Acute hypoxaemic respiratory failure is one of the most frequent common causes for admission to intensive care units (ICUs).1 The aim of non-invasive oxygen strategies is not only to improve oxygenation, but also to reduce inspiratory effort and potential related lung injury,2 and thus to allow time for the underlying disease to be treated without the need for intubation, sedation and invasive mechanical ventilation. The use of high-flow nasal cannula oxygen therapy (high-flow nasal oxygen) is recommended rather than standard oxygen as first-line therapy, because it reduces work of breathing and dyspnoea, and may reduce the risk of intubation.3 4 However, the superiority of high-flow nasal oxygen over standard oxygen for reducing mortality is not established, even with widespread use in ICU the last decade, especially during the COVID-19 pandemic.5–7 The benefits of high-flow nasal oxygen were reported in a first clinical trial published in 2015, with a reduction in mortality rates compared with other oxygen supports, and also a reduction of intubation rates in patients with moderate-to-severe hypoxaemia.1 By contrast, high-flow nasal oxygen has not been shown to be superior over standard oxygen in terms of either intubation or mortality rates in a specific population of immunocompromised patients.8–10 In patients with acute respiratory failure due to COVID-19, two clinical trials showed a decreased risk of intubation with high-flow oxygen as compared with standard oxygen,11 12 whereas other studies did not show any difference in the risk of intubation or mortality.13 14
As a result, recent clinical practice guidelines highlighted the need to identify patients who can benefit from high-flow nasal oxygen, as well as the need to explore the effects of high-flow nasal oxygen versus standard oxygen on mortality.4
Objectives
We are proposing a multicentre randomised controlled trial comparing high-flow nasal oxygen versus standard oxygen in patients admitted to ICU with acute respiratory failure and moderate-to-severe hypoxaemia, with the hypothesis that high-flow oxygen could reduce mortality.
Primary objective
To compare mortality 28 days after randomisation between an oxygenation strategy using high-flow nasal oxygen or standard oxygen.
Secondary objectives
To compare between the two groups:
Time to death between randomisation and day 28.
Intubation rates at day 28.
Numbers of ventilator-free days at day 28 is defined as the number of days alive without invasive mechanical ventilation between randomisation (day 1) and day 28.
Length of stay in ICU and hospital.
Mortality rates in ICU, in hospital and up until day 90.
Levels of oxygenation, comfort and grade of dyspnoea 1 hour after treatment initiation.
Time from randomisation to meeting intubation criteria and from randomisation to intubation.
Severity defined by the Sequential-related Organ Failure Assessment (SOFA) score during the first 48 hours after intubation.
Number of complications during ICU stay.
Methods: participants, interventions and outcomes
Trial design
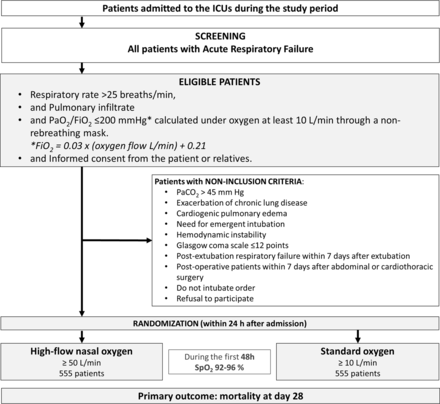
The SOHO trial (acronym of Standard Oxygen versus High-flow nasal Oxygen in patients with acute hypoxaemic respiratory failure) is an investigator-initiated, multicentre, open-label, randomised clinical trial comparing two strategies of oxygenation, high-flow nasal oxygen versus standard oxygen, in patients admitted to ICU for acute respiratory failure with moderate-to-severe hypoxaemia. Patients are randomly assigned to one of the two groups with a ratio of 1:1 (figure 1).
Consort diagram of the Standard Oxygen versus High-flow nasal Oxygen trial. ICU, intensive care unit; PaO2/FiO2, partial pressure of arterial oxygen to fractional inspired oxygen ratio; SpO2, pulse oximetry.
The trial is taking place in 41 ICUs in France, and centres in Spain are expected to participate. The SOHO trial started on 19 January 2021, while a wave of COVID-19 was occurring. Therefore, we stopped the original trial and decided to conduct an ancillary study exclusively focused on patients with acute respiratory failure due to COVID-19 (SOHO-COVID trial, NCT04468126, version 42021-04-29). This ancillary study started on 27 April 2021 and was completed on 6 December 2021. The original trial was then resumed. After the results of the ancillary SOHO-COVID trial showing decreased risk of intubation with high-flow nasal oxygen compared with standard oxygen were known, as of 8 September 2022 patients with acute respiratory failure due to COVID-19 were excluded. The patients included in this ancillary study will not be included in the original SOHO trial.
Eligibility criteria
Inclusion criteria
All consecutive patients older than 18 years, admitted to ICU with a hypoxaemic acute respiratory failure are eligible if they meet all the following criteria: respiratory rate >25 breaths/min, a pulmonary infiltrate on chest X-ray and a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ≤200 mm Hg while breathing oxygen at a flow of 10 L/min or more through a non-rebreathing mask (figure 1). For the calculation of PaO2/FiO2 ratio under standard oxygen, FiO2 is estimated as follows: FiO2=0.03×(oxygen flow L/min)+0.21.1 15
Exclusion criteria
Patients with the following criteria are not included: hypercapnia defined as PaCO2 >45 mm Hg, exacerbation of chronic obstructive pulmonary disease (grade 3 or 4 of Gold classification) or another chronic lung disease with long-term oxygen or ventilatory support, need for emergent intubation (including cardiac arrest, respiratory arrest or pulse oximetry (SpO2) <90% despite maximum oxygen support), cardiogenic pulmonary oedema as main reason for acute respiratory failure, haemodynamic instability defined by signs of hypoperfusion (mottling, cyanosis) or use of vasopressors >0.3 µg/kg/min, Glasgow coma scale <12 points, postextubation respiratory failure within 7 days after extubation, postoperative patients within 7 days after abdominal or cardiothoracic surgery, do not intubate order, patients without health insurance coverage, patient under protection (pregnant or breastfeeding women, minor patients, subjects with guardianship or under law protection) or refusal to participate. Acute respiratory failure due to COVID-19 infection was secondarily added (8 September 2022) as an exclusion criteria after the publication of the SOHO-COVID trial showing a decreased intubation rate in patients treated by high-flow nasal oxygen.11
Intervention
Patients eligible for inclusion are randomly assigned to the standard oxygen group (control group) or the high-flow nasal oxygen group (experimental group) and treatment is required within 3 hours after fulfilment of inclusion criteria.
Control group: standard oxygen
In the standard oxygen group, oxygen is delivered through facemask or non-rebreathing mask, with oxygen flow set at 10 L/min minimum, adjusted to maintain SpO2 between 92% and 96%, for at least 48 hours until the patient recovers, unless the patient requires intubation. In the absence of signs of respiratory failure 48 hours after treatment initiation (respiratory rate ≤25 breaths/min and SpO2 ≥92% under oxygen flow ≤6 L/min), standard oxygen is switched from facemask to nasal cannula. In case of persistence of respiratory failure symptoms 48 hours after treatment initiation, treatment is continued by periods of 24 hours until complete respiratory recovery or intubation.
Experimental group: high-flow oxygen
In the high-flow nasal oxygen group, high-flow nasal cannula oxygen therapy is delivered with oxygen applied through a heated humidifier (MR850, Fisher and Paykel Healthcare) continuously through large-bore binasal prongs, with a gas flow rate of at least 50 L/min and FiO2 titration of 5%–10% set to maintain SpO2 between 92% and 96% (Optiflow or Airvo-2, Fisher and Paykel Healthcare; or dedicated ICU-ventilator with high-flow oxygen therapy option) for at least 48 hours until the patient recovers, unless the patient requires intubation.
In the absence of signs of respiratory failure 48 hours after treatment initiation (respiratory rate ≤25 breaths/min and SpO2 ≥92% under a FiO2 ≤40%), high-flow nasal oxygen is stopped and switched to standard oxygen.16 In case of persistent respiratory failure symptoms 48 hours after treatment initiation, treatment is continued by periods of 24 hours until complete respiratory recovery or intubation.
Events during allocated oxygen strategy
Intolerance of oxygenation strategy
In case of intolerance to allocated strategy, gas flow is decreased in both groups, after which temperature can be reduced in the high-flow oxygen group,17 and resumed later if possible, except if the patient meets the criteria of intubation. In any case, the patients are included in the intention-to-treat analysis.
Rescue therapy
In case of respiratory worsening under allocated strategy, use of non-invasive ventilation (NIV) is discouraged. However, if investigators decide to apply NIV, ventilatory setting and expired tidal volumes should be closely monitored with a target between 6 and 8 mL/kg of predicted body weight. Intubation should be decided in case of persistent criteria of intubation more than 1 hour after initiation of NIV.
Prespecified criteria for intubation
To ensure the consistency of indications across sites and reduce the risk of delayed intubation, patients are immediately intubated if one of the following criteria occurs: severe respiratory failure; threatening hypoxaemia (recurrent episodes of SpO2 <80% or persisting SpO2 <88% with maximal oxygen support); cardiac arrest; haemodynamic instability with signs of hypoperfusion (mottling, cyanosis); altered consciousness (Glasgow coma scale <12). Severe respiratory failure leading to intubation is defined by at least two of the following criteria: (1) respiratory rate >40 cycles/min, (2) appearance or worsening of signs of respiratory-muscle fatigue, (3) acidosis with pH <7.35, (4) hypoxaemia defined as PaO2/FiO2 ratio <100 mm Hg or need for oxygen flow at least 15 L/min or FiO2 at least 80% to maintain a SpO2 ≥92%.
Outcomes
Primary outcome measure
The primary outcome is mortality 28 days after randomisation.
Secondary outcome measures
Secondary outcome variables include the following:
Time to death between randomisation and day 28.
The proportion of patients intubated at day 28.
The number of ventilator-free days at day 28, defined as the number of days alive without invasive mechanical ventilation between randomisation (day 1) and day 28.
Length of stay in ICU and hospital.
Mortality in ICU, in hospital and up until day 90.
Levels of oxygenation assessed by arterial blood gas sample, comfort assessed using a 100 mm visual analogue scale (0 indicating no discomfort to 100 mm indicating maximal imaginable discomfort), dyspnoea evaluated using a 5-point Likert scale model indicating marked improvement, slight improvement, no change, slight deterioration, and marked deterioration.
Interval between randomisation and meeting of intubation criteria and interval between randomisation and intubation.
Severity evaluated by SOFA score during the first 48 hours after intubation.
Complications during ICU stay including septic shock, nosocomial pneumonia, cardiac arrhythmia and cardiac arrest.
Participant timeline
Figure 1 shows the flow chart of the patients and the study design, and table 1 provides a detailed participant time line.
Study flow chart
Sample size
In accordance with the literature and our previous study,1 18 we determined that randomisation of 1110 patients would provide a power of 80% (beta risk of 0.2) to show an absolute difference of 6% in rate of mortality at day 28, between the control groups using standard oxygen (mortality rate estimated at 18%) and the experimental group using high-flow nasal oxygen (mortality rate estimated at 12%), at two-sided alpha level of 0.05.
Recruitment
The initial planned duration of patient recruitment is 4 years, with the SOHO trial starting on 19 January 19, 2021.
End of 2020: national grant award
2020–2021: approvals from ethics committee and trial tool development (electronic case-report form (e-CRF), randomisation system), participating centres opening and training.
2021–2025: inclusion of patients (the first participant was enrolled 19 January 2021).
2021–2022: start and end of inclusions in the ancillary SOHO-COVID trial, publication of results.11
2024–2025: end of inclusions, monitoring of participating centres and queries to investigators; overseen by the steering committee at the REVA network meetings every 6 months; blind review to determine protocol violation, to define intention-to-treat and per-protocol analysis populations; new queries to investigators, cleaning and closure of the database.
2026: data analysis, writing of the manuscript and submission for publication.
Methods: assignment of intervention
Allocation and sequence generation
A computer-generated randomisation in permuted blocks of four (unknown to investigators) is performed with stratification according to the country and immunosuppression status. Within the first 3 hours following validation of inclusion criteria, using a centralised web-based management system, patients will be randomly assigned in a 1:1 ratio to the high-flow nasal oxygen or the standard oxygen group.
Immunosuppression is defined as follows: use of long-term steroids (>3 months) or high-dose steroids (≥20 mg/day of prednisone or equivalent for at least 14 days), use of other immunosuppressant/immunomodulatory drugs, solid organ transplant, active solid cancer, haematological malignancy (active or remitting for less than 5 years), leucopenia <1 G/L or neutropenia ≤0.5 G/L after chemotherapy, allogenic stem cell transplantation within the last 5 years, AIDS or primary immune deficiency.10
Blinding
Although individual patient assignments cannot be masked, the coordinating centre and all the investigators will remain unaware of the study group outcomes until the data is locked.
An independent biostatistician, who will be unaware of study outcomes and treatment allocation, will collect patient data from the recordings and conduct analyses.
Method: data collection, management and analysis
Data collection and management
Data are collected on an e-CRF by a trained investigator or research assistant at each centre. Patient follow-up and data collected are detailed in the study flow chart (table 1).
Statistical methods
All the analyses will be performed by the study statistician, in accordance with the International Conference on Harmonisation and Good Clinical Practice guidelines. A predefined statistical analysis plan will be followed. All the analyses will be conducted by the biostatistics department of the Poitiers University Hospital using statistical software (SAS, V.9.3; SAS Institute; Cary; North Carolina, USA, and R V.2.14.1). The analysis will be performed on an intention-to-treat basis after validation by a blind review committee of the inclusion and exclusion criteria for each patient. A two-tailed p value of less than 0.05 will be considered as indicating statistical significance.
Descriptive analysis of patient groups at baseline
Continuous variables will be summarised with the classic parameters of descriptive analysis (median, IQR and extreme values or mean and SD), while indicating the number of missing data. Categorical variables will be presented in the form of absolute frequency and percentage in each modality.
Eligibility criteria will be verified based on the data recorded in the case reports. After meeting of the blind review committee, deviations from the protocol will be described and analysed on a case-by-case basis, and wrongly included subjects such as those lost to follow-up will be described.
Analysis pertaining to the main criteria of evaluation
The proportion of patients having died 28 days after randomisation (primary outcome) will be compared between the two groups by means of the χ2 test. Non-adjusted χ2 test will be the main criterion of evaluation to compare the primary outcome between the two groups.
A multivariate logistic regression will be performed to adjust on the stratification variables (immunosuppression status and country) and on the potential unbalanced baseline variables. Interactions will be tested before this multivariate analysis. The variables associated with day-28 mortality with a p value <0.20 will be considered in a maximal model and a backward-selection procedure will be performed. The final model will include variables significantly associated with day-28 mortality with a p value of less than 0.05. Results will be expressed as OR and 95% CI. To take into account a potential study centre effect, a mixed effects logistic regression model will be performed.
Analysis pertaining to the secondary criteria of evaluation
Kaplan-Meier curves will be plotted to assess time from randomisation to death and will be compared by means of the log-rank test at day 28 and at day 90. The percentages of patients having needed intubation at day 28, such as other qualitative outcomes (death in ICU, death in hospital, complications during ICU stay) will be compared between the two groups by means of the χ2 test. Kaplan-Meier curves will be plotted to assess time from randomisation to intubation and will be compared by means of the log-rank test at day 28. An adjusted HR with 95% CI will be calculated by a Cox proportional-hazard multivariate regression.
Lengths of stay in ICU and in hospital, ventilator-free days at day 28 and time to intubation will be expressed in median (IQR) and compared between the two treatment groups by means of the Mann-Whitney U test.
Levels of oxygenation, comfort, dyspnoea and SOFA score will be compared between the two treatment groups by means of the Student’s t-test.
Number of complications during the ICU stay will be compared using a Poisson regression.
Predetermined subgroup analysis
Analyses will determine if there exists an interaction between treatment effect and subgroups of patients, that is, immunocompromised and non-immunocompromised patients. A subgroup analysis will then be performed for main and secondary criteria of evaluation in immunocompromised and non-immunocompromised patients. Similarly, a subgroup analysis will be performed according to the severity of oxygenation at baseline, determined by the PaO2/FiO2 ratio (> and ≤100 mm Hg).
Methods: monitoring
Data monitoring
Before starting patient enrolment, all physicians and other healthcare workers in the ICU attend formal training sessions on the study protocol and data collection. An investigator at each centre is responsible for daily patient screening, enrolling patients in the study, ensuring adherence to the protocol and completing the e-CRF. Research assistants will regularly monitor all the centres on site to check adherence to the protocol and the accuracy of the data recorded.
Auditing
According to the French law, no safety committee is required because the interventions used in the study are strategies of oxygenation that are typically used in clinical practice.
Ethics and dissemination
The study has been approved by the central ethics committee (Ethics Committee Sud Méditerranée III) with the registration number 2020.07.05 (24 September 2020).
Consent or assent
Patients are included after having provided written informed consent to the investigator according to the decision of the central ethics committee. If the patient is not able to understand the information given, he/she can be included if the same procedure is completed with a next of kin. After the patient’s recovery, he/she will be asked if he/she agrees to continue the trial.
Confidentiality
Data are handled according to French law. Coding subjects are done by recording the first letter of the name and forename, accompanied by a single study identifier indicating the order of subject inclusion, in order to store anonymised data in the e-CRF. The sponsor will ensure that each study participant has given his/her consent for access to his/her personal data that is strictly required for quality control of the study. All original records will be archived at trial sites for 15 years.
Declaration of interest
The SOHO trial is an investigator-initiated trial funded by the French Ministry of Health obtained in 2019 from a national hospital clinical research programme (Programme Hospitalier de Recherche Clinique National 2019). A scientific committee including J-PF, RC and AWT conceived, drafted and wrote the project. The European research network REVA has endorsed the study project. The study is promoted by the University Hospital of Poitiers. The Fisher & Paykel Healthcare firm contributed to the funding of the project but has no other involvement in the study.
Access to data
All investigators will have access to the final data set. Investigators will make available the documents and individual data strictly required for monitoring, quality control and audit of the study to persons having access to them, in accordance with the statutory and regulatory provisions in place (articles L.1121-3 and R.5121-13 of the French Public Health Code).
Dissemination policy
Findings will be published in peer-reviewed journals and presented at national and international meetings. Communications, reports and publication of the results of the study will be placed under the responsibility of the principal investigator-coordinator of the study and the executive committee. Rules of publication will follow the international recommendations according to The Uniform Requirements for Manuscripts (ICMJE, April 2010).
Patient and public involvement
Patients and public are not involved in the study.
Discussion
While standard oxygen is the first-line therapy for acute hypoxaemic respiratory failure, high rates of intubation failure ranging from 30% to 50% and mortality ranging from 11% to 30% have led to the development of other oxygenation strategies, that is, high-flow nasal oxygen and NIV, to bridge the therapy gap between standard oxygen therapy and invasive mechanical ventilation.1 10 11 13 19
Although NIV has beneficial physiological effects such as reduction in inspiratory effort,20 it may be deleterious in patients with acute hypoxaemic respiratory failure by providing large tidal volumes and by promoting patient self-inflicted lung injury, as in cases of acute respiratory distress syndrome.4 21–23 In previous clinical practice guidelines, no specific recommendations have been made for or against the use of NIV in the management of patients with acute hypoxaemic respiratory failure.4 21 Consequently, NIV was not chosen, either in the control group or in the experimental group of the SOHO trial.
Paradoxically, the clinical benefits of high-flow nasal oxygen have been reported before exploration of its physiological effects, which include delivery of high fraction of oxygen, generation of positive end expiratory pressure effect, washout of the upper airway with ultimately a decreased work of breathing.3 24 25 A seminal trial comparing high-flow nasal oxygen with standard oxygen and NIV in patients with acute hypoxaemic respiratory failure showed decreased mortality rates in the high-flow oxygen group, and decreased intubation rates in the most hypoxaemic patients.1 However, another large trial comparing high-flow nasal oxygen with standard oxygen in immunocompromised patients did not confirm the benefits of high-flow nasal oxygen, with no significant difference in terms of intubation or mortality between the two groups.10 In patients having acute respiratory failure due to COVID-19, several trials showed a reduction of intubation rates with high-flow nasal oxygen as compared with standard oxygen, especially in severe patients.11–13 However, these trials failed to show any significant reduction in mortality rates with high-flow nasal oxygen. Although the most recent guidelines recommend high-flow nasal oxygen as first-line therapy over standard oxygen in acute hypoxaemic respiratory failure insofar as it reduces the risk of intubation, its effect in reducing the risk of mortality is not proven.4 Accordingly, future trials are needed to confirm the superiority of high-flow nasal oxygen over standard oxygen,4 and for this reason high-flow nasal oxygen has been chosen as the experimental treatment in the SOHO trial.
The population of the SOHO trial includes moderate-to-severe hypoxaemic patients with or without immunosuppression status. Moderate-to-severe hypoxaemia is defined by a PaO2/FiO2 ratio equal to or below 200 mm Hg, which corresponds to high risk of intubation in our previous study.1 22 As immunocompromised patients with acute respiratory failure have a higher mortality rate than non-immunocompromised patients,8 26–28 stratification will be performed on this variable at randomisation, to ensure balanced distribution of immunocompromised patients in the two groups of treatments. Analyses will determine if there exists an interaction between treatment effect and patient subgroups.
Consequently, the primary outcome is mortality at day 28, an objective patient-important outcome,29 while intubation is more subjective, even if prespecified criteria for intubation are applied. Compared with mortality at day 90, mortality at day 28 is more closely related to the stay in intensive care and less closely to the underlying diseases, as in immunocompromised patients.
In conclusion, the SOHO trial is an investigator-initiated multicentre, open-label, randomised clinical trial empowered to test the hypothesis that high-flow nasal oxygen in comparison with standard oxygen helps to reduce the rate of mortality in patients with acute hypoxaemic respiratory failure. The SOHO trial will also assess the impact of high-flow and standard oxygen on intubation rates, blood oxygenation, comfort and complications during ICU stay.
Ethics statements
Patient consent for publication
References
Footnotes
X @Quenot, @AF_Dureau
Collaborators REVA network: Jean-Pierre Frat, Rémi Coudroy, Christophe Guitton, Stephan Ehrmann, Alexandre Demoule, Guillaume Carteaux, Damien Contoux, François Beloncle, Jean-Christophe Richard, Gaël Bourdin.
Contributors J-PF accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish. J-PF, RC and AWT designed together the study, and wrote together the manuscript. SR provided substantial contributions to the conception and design of the study, and wrote with J-PF the statistical analysis plan and estimated the sample size. J-PF, RC, SR and AWT contributed for drafting the work, revising it critically for important intellectual content and approved the final version of the manuscript. All authors give their agreement to be accountable for all aspects of the work, and ensure the accuracy and integrity of any part of the work.
Funding This study was funded by Ministère des Affaires Sociales et de la Santé (PHRC-19-0305), Fisher and Paykel Healthcare (contribution to financing the study). The study promoter is the University Hospital of Poitiers, Poitiers, France. The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer The firm Fisher and Paykel Healthcare contributed to financing the study but has no other involvement in the study.
Competing interests J-PF reports grants from the French Ministry of Health; personal fees for lectures, travel expense coverage to attend scientific meetings, grant for randomised clinical trial from Fisher and Paykel Healthcare; personal fees as member of a scientific board and travel expense coverage to attend scientific meetings from SOS Oxygène outside this work. RC reports grants from the European Respiratory Society and the French Intensive Care Society, and travel expense coverage to attend scientific meetings from Fisher and Paykel Healthcare and MSD. SE discloses consultancies from Aerogen, research support, speaker fees and travel support from Aerogen and Fisher & Paykel Healthcare, research support from Open AI. AD reports grants from the French Ministry of Health, Assistance publique – Hôpitaux de Paris, Lungpacer, Respinor, consulting fees from Respinor, Lungpacer, Lowenstein, Tribunal administratif de Cergy, Liberate Medical, payment or honoraria for lectures, presentations from Fisher & Paykel, Baxter, Getinge, Astra, Agence Européenne Informatique, Mindray, support for attending meetings and/or travel from Lungpacer, outside the submitted work. GCarteaux reports personal fees from Air Liquide Medical System, GE Healthcare, Dräger, Fisher and Paykel, Medtronic and Löwenstein, outside the submitted work. FB reports consulting fees from Löwenstein Medical and Air Liquid Medical Systems and research support from Covidien and GE Healthcare outside this work. J-CR reports grant from Hamilton medical and travel expense coverage to attend scientific meetings from GILEAD and Pfizer. GH has received personal fees for lectures, travel expense coverage to attend scientific meetings from Fisher and Paykel Healthcare. AWT reports travel expanse coverage to attend scientific meetings and payment for lectures from Fisher and Paykel Healthcare, Covidien, Maquet-Getinge, Dräger Medical, General Electric Healthcare.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.