Article Text
Abstract
Introduction Diminished levels of meaning in life can have a range of detrimental effects on cancer patients, including heightened anxiety, depression, psychological distress, reduced quality of life and, in severe cases, even thoughts of suicide. Notably, young and middle-aged cancer patients often exhibit even lower levels of meaning in life compared with their counterparts in other age groups. The primary objective of this study is to formulate a meaning in life intervention programme and assess its efficacy in enhancing the meaning in life and other relevant indicators among young and middle-aged cancer patients.
Methods and analysis A prospective, parallel-group randomised controlled trial will be conducted. Eighty-eight young and middle-aged cancer patients will be randomised into either the intervention or control group. The intervention group will receive 4 week, six-session, group-based meaning in life intervention programme, while the control group will receive treatment as usual. The primary outcome is meaning in life, and secondary outcomes are post-traumatic growth and psychological distress. These indicators will be assessed at baseline, on completion of the intervention and again 2 months following its conclusion.
Ethics and dissemination The trial has received approval from the Institutional Review Board of Shanghai Proton and Heavy Ion Hospital (2202-53-04-2301A-2310B). The study results will be shared through peer-reviewed journals and conferences.
Trial registration number Chinese Clinical Trial Registry, ChiCTR2200060672.
- Randomized Controlled Trial
- ONCOLOGY
- Protocols & guidelines
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Methodological rigour, encompassing practices such as concealed random allocation, blinded outcome assessment and the prospective registration, is expected to mitigate the risk of bias.
The intervention is theory-based and has been developed and refined based on previous research findings and qualitative data collected from young and middle-aged cancer patients.
Due to the nature of the psychological intervention, neither the participants nor the interventionists will be blinded.
Only the short-term follow-up effects for 2 months after the intervention will be studied.
One of the limitations of the study is that all outcomes will be measured through self-report.
Introduction
In 2020, approximately 19.3 million new cancer cases were reported.1 Projections suggest that by 2040, global cancer cases will rise to 28.4 million, reflecting a substantial 47% increase compared with 2020. 1 Notably, 23.7% of all newly diagnosed cancer cases occur in China.2 As the world’s largest developing country, China shoulders a substantial burden of cancer. As the number of cancer cases gradually increases, an expanding body of research reveals a concerning trend: the incidence of various types of cancers, including head and neck, colorectal, breast, pancreatic, kidney and uterine cancers, among others, is steadily rising among young-middle aged individuals.3–5
Young and middle-aged individuals often hold important familial and social roles,5 and being diagnosed with cancer subjects them not only to physical discomfort but also to psychological distress and existential challenges. These challenges encompass an array of struggles, such as the inability to fulfil family roles, disruptions in the sequence of significant life events, difficulty in achieving life goals, uncertainty about the future and regrets concerning unfulfilled aspirations.6 Additionally, compared with other age groups, they face greater challenges related to illness, life, work and financial pressures,7 8 which may lead to a diminished sense of meaning in life or even a complete loss of it.9 10 Several studies have consistently confirmed that the level of meaning in life of young and middle-aged cancer patients is notably low or pessimistic.11–13
Emphasising the significance of meaning in life throughout their treatment, recovery and ongoing mental health maintenance proves to be immensely valuable.14 The meaning in life plays a crucial role in alleviating anxiety, depression and distress, and nurturing a sense of meaning can significantly enhance the quality of life and overall physical and mental health.15–17 Moreover, cancer patients often express the importance of seeking meaning and purpose in their lives.18 Conversely, patients experiencing a diminished sense of meaning are prone to lower survival rates and higher suicide rates.19 20 These findings underscore the imperative to intervene in the meaning in life for cancer patients, particularly young and middle-aged ones.
Meaning-centred psychotherapy is a psychotherapeutic intervention rooted in Frankl’s logotherapy, specifically designed to cater to the psychological needs of advanced cancer patients.21 It has been further adapted and applied across various cultural contexts and diverse populations.22–29 In the initial stages, our group carefully revised the original meaning-centred group psychotherapy (MCGP), considering both Chinese culture and the specific characteristics of the target population. Subsequently, we conducted a randomised controlled trial and observed a positive impact on enhancing the meaning in life of Chinese cancer patients undergoing active treatment.29 However, during the practical application and based on feedback from participants, we identified areas for improvement in the programme, including diversifying the presentation of themes to accommodate varying levels of insight among patients, ensuring participants are of similar age to optimise their engagement experience and better scheduling group sessions to reduce conflicts with treatment.30
Hence, the primary objective of this study is to formulate an intervention programme aimed at enriching the meaning in life. This endeavour was guided by the principles of logotherapy theory and built on the groundwork laid by the research team’s prior experience. We hypothesise that a 4 week, 6-session meaning in life intervention programme will effectively improve the meaning in life, foster post-traumatic growth, and alleviate psychological distress among young and middle-aged cancer patients.
Methods
Design
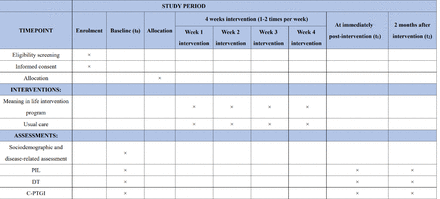
This two-arm, parallel-design randomised controlled trial among young and middle-aged cancer patients will follow the Consolidated Standards of Reporting Trials flow chart31 (see figure 1). The SPIRIT checklist is available as a supplementary document32 (online supplemental material 1). Eligible patients who consent to participate will be randomly assigned to either the intervention group or the control group. The intervention programme comprises a total of 6 themes and spans 4 weeks. The primary outcome, meaning in life and secondary outcomes, including post-traumatic growth and psychological distress, will be evaluated at three time points: baseline, post-intervention and 2 months post-intervention. The assessment timeline is visually depicted in figure 2. The study will commence in September 2023 and is expected to conclude by March 2024.
Supplemental material
CONSORT flow chart of the study.
The schedule of trial enrolment, interventions and assessments. C-PTGI, Chinese version of the Post-Traumatic Growth Inventory; DT, Distress Thermometer; PIL, Purpose in Life Test.
Study setting
This study will be conducted at the Shanghai Proton and Heavy Ion Centre, where proton and heavy ion radiation therapy are the primary treatment modalities. As of 8 May 2023, a cumulative total of 5648 patients have received treatment and have been discharged from the hospital, demonstrating an average annual growth rate of 20%.
Recruitment
Participants will be recruited from September 2023. Recruitment will be conducted through various channels, including public advertisements (eg, posters, flyers) and mobilisation meetings (inform potential participants about the risks associated with a low sense of meaning in life and the content and benefits of this project). Additionally, word-of-mouth dissemination by nurses about the project will be used to reach potential participants. Participants who meet the inclusion and exclusion criteria and voluntarily choose to participate will receive comprehensive information from the investigator. This information will cover the study’s purpose, procedures, interventions, assessment details, randomisation procedures, potential risks, anticipated benefits, data confidentiality and an explanation of their rights as subjects. Upon agreement, participants will provide written informed consent (online supplemental material 1). They will be informed that they can withdraw from the study at any time without impacting their treatment.
Participants
The inclusion criteria for participants are as follows: (a) inpatients diagnosed with any type of solid tumour, (b) aged between 20 and 49 years, (c) aware of their diagnosis, (d) fluent in Mandarin and (e) voluntarily participated in this study by signing the informed consent form. The age range of 20–49 years was chosen based on the definition of early-onset cancer,33 and research indicates that the meaning in life among cancer patients under 50 is often concerning.11–13 The exclusion criteria are as follows: (a) individuals in the acute phase of the disease or experiencing severe hepatic, renal or cardiopulmonary impairment, (b) those with severe mental disorders or significant cognitive deficits and (c) individuals currently taking psychiatric medications or engaged in other psychological interventions.
Sample size
The sample size was determined using Power Analysis and Sample Size Software (PASS, V.15.0.5). Specifically, based on a two-sample t-test at a two-sided significance level of 0.05, the study is designed to achieve a power of 90% in detecting the anticipated effect. The sample size calculations are based on the primary outcome (meaning in life), using an effect size of 0.78 for the primary end point of change from baseline to post-intervention in the Purpose in Life Test (PIL), as evidenced in a previous study we conducted.29 Consequently, a sample size of 72 participants is deemed necessary to detect this effect. Accounting for an estimated dropout rate of 20%, a minimum of 88 subjects needed to be recruited for the study.
Randomisation and blinding
The study will employ block randomisation with four block sizes and a 1:1 allocation ratio. The random sequence will be generated using the computer software Study Randomiser (https://www.studyrandomizer.com/) by a graduate student who is not involved in recruitment, intervention or data collection. The grouping scheme for each block will be sequentially placed in four small opaque sealed envelopes, each numbered from 1 to 4. Subsequently, these envelopes will be uniformly placed in a large envelope labelled with the corresponding block number. Throughout the study, all envelopes will be kept individually. To ensure minimal selection bias, the generation of the allocation sequence will be completed before the recruitment phase of the study begins. Upon enrolling patients who meet the inclusion criteria into this study, another researcher will sequentially open the envelopes to obtain grouping information for allocation concealment. To uphold blinding, data collectors and data analysts will remain unaware of the grouping information.
Intervention: meaning in life intervention programme
Cancer patients in the intervention group will receive the meaning in life intervention programme. Considering the duration of a patient’s hospital stay, approximately 1 month, we have aligned the duration of the intervention programme accordingly. While many intervention programmes designed to improve the meaning in life for cancer patients typically have eight sessions, our practical experience has shown that balancing the requirement for eight sessions in 1 month with each patient’s treatment schedule can be a challenge. A previous study showed that patients who fully participated in the intervention achieved greater improvements in their meaning in life compared with those who did not fully adhere to the intervention.29 Hence, to enhance patient participation, we will opt to schedule six sessions spread across 1 month. This approach significantly increased scheduling flexibility and substantially reduced the risk of patients missing the programme due to conflicting treatment and intervention schedules.
The meaning in life intervention programme is grounded in logotherapy theory, drawing on insights from the MCGP and the book 'GUIDEPOSTS to MEANING: Discovering what really matters'.34" In the intervention group, patients will engage in a 4 week, 6-session meaning in life intervention programme, lasting 2 hours per session, conducted twice a week. Each session introduces participants to distinct themes, such as ‘Value’, ‘Historical Sources of Meaning’, ‘Attitudinal Sources of Meaning’, ‘Free Choice and Responsibility’, ‘Creative Sources of Meaning’ and ‘Experiential Sources of Meaning’ (online supplemental material 1). Each session comprises 10 min of didactics, followed by 10 min of mindfulness meditation and 40 min of experiential exercises, and concludes with a 60 min group discussion. Each experiential exercise will be introduced as a game or visualisation. During the discussion phase, the interventionist will pose guiding questions tailored to each patient’s result from the exercise, aiming to accomplish the objectives of each topic. The group will be co-led by two group psychotherapists trained in meaning-centred therapy and a doctoral student in nursing psychology. Each closed group will comprise approximately 10 participants. During the trial, participants in the intervention group will receive two face-to-face reminders and notifications from their charge nurse before each group activity: one day in advance and another 2 hours prior to the scheduled start time.
Control group
Patients will receive standard care throughout their hospitalisation, including nutritional support, rehabilitative care and routine psychosocial care. This routine psychosocial care involved systematic mental health assessments conducted by nurses. If deemed necessary, trained nurses will provide non-specific psychological support, including empathetic interactions, encouragement and opportunities for patients to share their thoughts and feelings.
Intervention fidelity
The evaluation of intervention implementation effectiveness will involve recording audio from all group sessions. An expert with a background in both medicine and psychology will conduct a random selection of 20% of the tapes for reviewto assess the implementation of the intervention programme. Adherence to the study protocol will be defined as attending a minimum of five sessions, which will be closely monitored and documented by the interventionist. Any instances of the absence or withdrawal will be documented, and reasons will be provided. This process aims to gauge participant adherence to the therapeutic interventions.
Data collection
A graduate nursing student, equipped with training in systematic research, will assess the outcomes of the intervention for eligible participants. At baseline, sociodemographic and disease-related variables will be collected. Additionally, three psychosocial variables—meaning in life, post-traumatic growth and psychological distress—will be assessed at three time points: baseline, post-intervention and 2 months post-intervention. Both the control and intervention groups will undergo reassessment at the same intervals. The baseline and post-intervention data collection will occur face-to-face, while the 2 month post-intervention data collection will be conducted online using Questionnaire Star.
Outcome measures
Sociodemographic and disease-related variables
A sociodemographic questionnaire will be distributed at the baseline to assess various demographic characteristics, including age, gender, marital status, educational level, religion, employment status, annual income, whether they have children, whether they have commercial insurance and the financial burden of treatment costs. Additionally, information related to the disease will be collected, such as cancer site, primary/recurrence, duration of illness, cancer stage, family history, presence of comorbidities, history of chemotherapy, history of surgery and other treatment histories.
Primary outcome: meaning in life
The PIL35 was developed by Crumbaugh and others based on logotherapy and was later adapted into Chinese by Chinese scholars.36 The participants' meaning in life will be assessed using the Chinese version of the PIL. This scale comprises 20 items, distributed across four dimensions: life purpose, life feeling, life value and life attitude. Each item is rated on a seven-point Likert scale, resulting in a total score range of 20 to 140. A higher score indicates a stronger sense of meaning and purpose in life. In our preliminary research on young and middle-aged cancer patients, the PIL demonstrated a good Cronbach’s alpha coefficient of 0.869.29
Secondary outcomes
Post-traumatic growth
The Post-Traumatic Growth Inventory (PTGI)37 was developed by Tedeschi and others and was later adapted into Chinese by Chinese scholars.38 Post-traumatic growth will be assessed using the Chinese version of the Post-Traumatic Growth Inventory (C-PTGI). The scale consists of 20 items categorised into five adjusted dimensions: life perception, personal strength, new possibilities, relationships with others and self-transformation. It uses a six-point Likert scale, with scores ranging from 0 to 5 for each item and a total score ranging from 0 to 100. In our preliminary research on young and middle-aged cancer patients, the C-PTGI exhibited a strong Cronbach’s alpha coefficient of 0.873.29
Psychological distress
The distress thermometer (DT)39 was developed by Rosten et al. The Chinese version of the DT was translated by Chinese scholars and adjusted according to the actual conditions of Chinese cancer patients. Psychological distress will be assessed using the DT, where 0 indicates no psychological distress and 10 indicates extreme distress. The higher the score, the more severe the psychological stress. A DT of ≥4 indicates that the patient is experiencing clinically significant psychological distress and requires supportive services.40
Data analysis
An independent examiner will conduct the statistical analysis using IBM SPSS Statistics version 24.0 (IBM). All analyses will be carried out following an intention-to-treat approach. We will conduct a descriptive analysis of sociodemographic variables, disease-related variables, data missingness rates and all outcomes in young and middle-aged adults with cancer. For variables following a normal distribution, we will use means and SD; for those with non-normal distribution, medians and interquartile ranges (25th and 75th percentiles) will be employed. Categorical variables will be analysed through frequencies and percentages. Baseline characteristics of the two groups will be compared using either parametric or non-parametric tests. If the collected data follow a normal distribution, the analysis will involve Student’s t-test or χ2 test. In cases where the data do not meet normality assumptions, non-parametric tests such as the Wilcoxon test and Mann–Whitney U test will be employed.
A linear mixed model will be used to assess the impact of the intervention on primary and secondary outcome measures, including study group, time and the interaction between group and time as fixed effects, with participants as a random effect. Covariates integrated into the models encompass demographic variables that exhibit disparities between groups during baseline comparisons, along with those that exhibit a significant correlation with the pre- and post-intervention outcome indicator scores. Missing data will be addressed using multiple imputations with predictive mean matching,41 and sensitivity analyses will also be conducted. The significance level will be established at p<0.05.
Data management, monitoring and confidentiality
As the current intervention is non-pharmacological and adheres to the ethical principle of benefiting participants without causing harm, the likelihood of adverse effects due to the intervention is low. Consequently, establishing a Data Monitoring Safety Board appears unnecessary. All collected data are exclusively identified by a participant identification number to uphold confidentiality. Paper records, including informed consent forms and questionnaire data, will be securely stored in a locked office filing cabinet. Furthermore, audio recordings will be stored on a secure cloud server and safeguarded on a dedicated laptop computer. The management of the final trial dataset will primarily be handled by the first, second and final authors. Nonetheless, all authors will have access to the dataset if needed. We do not have any intentions of publicly releasing the individual-level dataset or any statistical code.
Adverse events
Throughout the entire study duration, we will meticulously document both anticipated and unanticipated adverse events. While we do not anticipate any serious adverse events, in the unlikely event of an unanticipated serious adverse occurrence (eg, a risk of suicide), all participants will have the opportunity to seek guidance and support from a specialist within the study team. Any notable adverse events will be promptly reported to the Institutional Review Board of Shanghai Proton and Heavy Ion Hospital.
Patient and public involvement
After implementing the MCGP with Chinese cancer patients, our research team conducted interviews with participants who had engaged in the programme. We incorporated the feedback and suggestions they offered into our current study, which encompassed the incorporation of positive thinking meditation exercises and the presentation of experiential exercises through gamification or visualisation techniques, among other enhancements.
Ethics and dissemination
The ethical committee of Shanghai Proton Heavy Ion Hospital has approved the entire study design (2202-53-04-2301A-2310B) and the informed consent forms. In the event of protocol changes, updates to trial registration information will be made accordingly. The results of this study will be included in a doctoral thesis by the lead author and subsequently submitted for publication in a peer-reviewed journal. If possible, the findings will also be presented at a relevant conference.
Discussion
The purpose of this study is to aid young and middle-aged cancer patients during active treatment in discovering potential sources of meaning through experiential exercises and open-ended discussions. By effectively using these sources, the patients could enrich their meaning in life, empowering them to embrace life to the fullest even after their discharge from the hospital. The outcomes of this randomised controlled trial are expected to offer significant and novel insights into the positive influence of the meaning in life intervention program on the meaning in life of young and middle-aged cancer patients.
Research has revealed that the age of a cancer patient can exert a substantial influence on their meaning in life, leading to diverse experiences and perspectives on this matter among different age groups.42 Patients within the same stage of life are more likely to connect and engage effectively in discussions and exchanges related to existential themes.30 Therefore, the meaning in life intervention programme offers a supportive environment for young and middle-aged cancer patients, enabling them to delve deep into existential issues that cancer presents, alongside exploring the distinctive psychological challenges associated with their age group, such as concerns about future goals, careers and family. Moreover, this intervention holds particular significance for young and middle-aged cancer patients, as the programme fosters patients' awareness of avenues to find meaning, enabling them to not only embrace and adapt to their illness while navigating multiple roles but also offer them a renewed sense of purpose and direction.
After the completion of the current project, our final focus will be on sustaining such projects in the long term. We are fully dedicated to crafting a comprehensive brochure, with the aim of ensuring its accessibility not only in specialised oncology hospitals but also in public hospitals, thus providing indispensable support to individuals grappling with cancer.
This study is expected to have some limitations. First, this study represents a single-centre randomised trial, and therefore, its findings may not be readily applicable to all settings. Further research conducted in a multi-centre, interdisciplinary and transregional context will be imperative. Second, considering the specificity and cost of treatment, the patients we recruit may be young and middle-aged cancer patients who meet the criteria for proton-carbon ion therapy and have higher incomes. This could potentially limit the generalisability of the study results. Finally, it should be noted that in our study, the control group receives standard care rather than an active control. The effectiveness of our intervention might be partly due to the non-specific effects of group therapy.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors SMW and HWW made significant contributions to the research methodology design and the development of the overall research objectives. WJX, YZ and MMZ participated in drafting the manuscript and providing critical input for important ideas. All authors collectively contributed to the final version. Each author played a comprehensive role in the project and assumed public responsibility for relevant portions of the content. The guarantor of the study is SMW who accepts full responsibility for the finished work and/or the conduct of the study, had access to the data and controlled the decision to publish.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.