Article Text
Abstract
Introduction Upper limb and core strength training is essential for older adults to safely perform daily activities. However, existing exercise programmes mainly focus on lower limb strength and are not designed or delivered to suit people with different functional capacities. This study describes the design of a two-arm cluster randomised controlled trial to examine the effects of a multicomponent physical activity (PA) programme, Mobility-Fit, on mobility and frailty in older adults living in care facilities.
Methods and analysis 160 older adults from 20 care facilities in Hong Kong will be recruited and randomised by care facilities (1:1) to an intervention or a control group. Participants in the intervention group will attend the Mobility-Fit programme, led by facility-based instructors, three times per week, 45 min per session, for 12 weeks, while the control group will participate in a standard care lower limb strengthening programme offered by the care facility. Participants will then be followed up for 9 months. Mobility-Fit comprises agility, postural coordination, balance and strength training, with suitable dosage based on participant’s baseline physical and cognitive function. The primary outcomes encompass upper and lower limb strength, trunk stability, reaction time, mobility function and fall efficacy. Secondary outcomes comprise daily PA level and performance, frailty, cognitive function and quality of life. A repeated measures analysis of variance (ANOVA) and generalised estimating equation (GEE) will be used to examine changes in outcomes over time and between groups. Data will be analysed following the intention-to-treat principles. We will also evaluate programme implementation and health economics throughout the follow-up period.
Ethics and dissemination Ethical approval was acquired in November 2022 from the Joint CUHK-NTEC Clinical Research Ethics Committee in Hong Kong (CREC-2022-459). Informed consent will be obtained from participants. The results of the study will be disseminated through peer-reviewed articles, conference presentations and social media.
Trial registration number ChiCTR2300072709.
- Aged
- Gait
- Nursing Care
- Posture
- Quality of Life
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The multicomponent programme addresses strength, balance, coordination and agility, which are important for activities of daily living and fall protective responses, and it has been tested for feasibility in frail older adults in care facilities.
The cluster randomised controlled design provides a rigorous means to examine the effectiveness of the multicomponent programme on safe mobility and reduces the likelihood of experimental contamination.
The measurement of daily activities with a wearable sensor allows the monitoring of physical performance in real-life context.
The programme delivery by facility-based instructors is expected to enhance the adherence and the sustainability of the Mobility-Fit programme.
The study is not powered to examine the effect of the programme on the frequency of fall-related injuries.
Introduction
It is estimated that people aged 65 and older are expected to make up 20%–25% of the world’s population By 2030.1 This profound ageing of the population brings about substantial challenges in providing adequate care for seniors, particularly those residing in care facilities,2 which serve as essential housing options for older adults who require assistance with activities of daily living (ADL).3 4 Over 50% of older adults in care facilities exhibit varying degrees of frailty that restricts their ability to perform basic daily tasks.5 Intriguingly, even among functionally ambulatory older adults in care facilities, it has been observed that they spend nearly 90% of waking time in sedentary behaviour.3 This sedentary lifestyle is of significant concern as low levels of physical activity (PA) have been strongly associated with mobility limitations, thereby increasing the risk of falls.3 6 Indeed, more than half of older adults in care facilities experience at least one fall per year, and most of these falls occur during routine activities such as walking, turning or transferring between positions.7 The high prevalence of falls during ADLs suggests the urgent need to promote safe mobility for older adults in care facilities to prevent them from falling and increase their independence.
The existing body of research suggests that PA and exercise programmes have the potential to preserve functional capacity and reduce the likelihood of nursing home placement for older adults.8 Notably, it has been reported that exercise could reduce the rate of falls by 23% in community-dwelling older adults.9 However, the effectiveness of exercise programmes in reducing falls within care facilities remains uncertain.10 Nevertheless, numerous studies have confirmed the promise of exercise interventions in slowing the progression of frailty and mobility impairment in frail older adults residing in care facilities.11 12
Furthermore, previous research has highlighted the importance of integrating ADLs into PA programmes for older adults in care facilities, since interventions involving the practice of daily activities, such as walking, transferring and dressing, led to improvements in ADL performance.13 Our scoping review has indicated that recreational PAs such as dancing, walking or active ball games can effectively enhance the mobility of older adults in care facilities.14 However, a limitation of many existing programmes is their focus on single-component exercises. Meanwhile, they often excluded frail or cognitively impaired older adults, who may require different components of training.14 Our previous research found that over 20% of falls in care facilities were caused by poor postural control during transferring,7 and one-third of falls involved head impact despite the use of upper arm arrests.7 15 These findings suggest that PA programmes for frail older adults should focus not only on balance and lower limb function but also on upper limb and core strength to enhance safe mobility and protective responses (eg, reach to grasp, hand arrest) to prevent fall-related injuries.
This research protocol addresses important issues by introducing the ‘Mobility-Fit’ programme, a multicomponent PA programme designed to promote safe mobility for older adults in care facilities.16 Adapted from the Osteofit programme, Mobility-Fit extends its focus beyond balance, quadriceps strength and coordination to incorporate upper limb and core strength training, aiming to enhance safe transferring and the ability to recover balance and prevent falls.7 15 17 Mobility-Fit specifically targets the strength and functional mobility aspects crucial for performing ADLs effectively. Importantly, the programme has shown promise in a pilot study conducted in care facilities, demonstrating its feasibility and ability to improve upper and lower limb strength, reaction time, and sit-to-stand performance, as well as in older adults with physical and cognitive impairment.16
Despite these encouraging preliminary results, a critical gap remains to be addressed. The effectiveness of this multicomponent programme has not been systematically compared with single-component programmes, such as lower limb strengthening, regarding their impact on functional mobility and trunk stability during transfers. Moreover, the potential effect of core strength training to enhance trunk stability,18 particularly in older adults residing in care facilities, remains largely unexplored. Given that over 30% of older adults in care facilities are pre-frail and 50% are frail, interventions aimed at engaging them in PAs to prevent the progression of frailty and mobility disability must be tailored to their specific functional capacities,19 and the adaptation mechanisms to exercise for those vulnerable older adults with varying functional capacities also need to be studies in depth.
Therefore, the first objective of this cluster randomised controlled trial (RCT) is to comprehensively examine the effects of the Mobility-Fit on strength, trunk stability, mobility, ADL performance and frailty in older adults living in care facilities; the effects will be compared with those of an existing lower limb strengthening programme. The second objective is to investigate the effectiveness of the Mobility-Fit programme in older adults with different degrees of frailty, mobility and cognitive function through subgroup analyses. The third objective is to evaluate intervention fidelity, sustained effects, programme delivery barriers and facilitators, and health economics of the Mobility-Fit programme throughout the 12-month study period.
Methods and analysis
Study design
The effects of Mobility-Fit programme in older adults resided in care facilities will be investigated in the two-arm cluster RCT. 20 clusters (care facilities) comprising approximately 8 participants per cluster will be randomly assigned to the intervention or the control group, which will receive an existing lower limb strengthening programme provided by the facilities as part of their standard care. The study will be carried out over a 12-week intervention, and a 9-month follow-up, with data collection occurring at baseline, 3 months (completion of the intervention) and 9 months after intervention (figure 1). This study protocol follows the Standard Protocol Items: Recommendations for Interventional Trials guidelines20 and has been registered on the Chinese Clinical Trial Registry (ChiCTR2300072709, summarised in table 1).
Trial registration information
Flow diagram of the study design.
Sample size calculation
The sample size was calculated with a priori power analysis using G*Power V.3.1.9.2. To achieve 90% power in detecting a moderate effect size (f=0.3) in outcome measures for the primary objective, we will require 80 participants (40 per group) with an alpha of 0.05 using repeated measures analysis of variance (ANOVA) and assuming a correlation among repeated measures of r=0.5. To account for the clustered nature of the data,21 we multiplied the estimated sample size by a design effect, which was calculated as 1+(n–1)×ρ, where n represents the cluster size and ρ stands for the anticipated intracluster correlation coefficient (ICC). Given the limited availability of prior studies for estimating the range of probable values for the ICC, we used a conservative estimate of 0.05, which is towards the upper end of the range observed in previous studies conducted in care facilities.22 Assuming that we can recruit approximately 8 participants per cluster, the design effect would be approximately 1.35, resulting in a sample size of 108 participants. We increased the sample size to address 25% potential loss to follow-up, which is based on previous studies conducted in care facilities.14 Additionally, we will further increase the sample size to account for subgroup analyses, which is predicated on the proportion of frail older adults in care facilities in Hong Kong.19 Accordingly, our final sample size will comprise 160 participants.
Study participants and eligibility criteria
Participants will be recruited between July 2023 and November 2023 from the care facilities operated by the Hong Kong Sheng Kung Hui (HKSKH) Multi-disciplinary Outreaching Support Team for the Elderly. Older adults will be eligible if they meet the following inclusion criteria: (1) older men and women (65 years or older) who reside in care facilities; (2) able to get up from a chair (with or without using the armrests) and stand for 20 s and (3) medically stable and approved by their physician.
Participants will be excluded if they meet the following criteria: (1) do not understand simple instructions; (2) receive treatment for medical conditions that preclude PA (eg, are bedridden) or (3) are legally blind. Eligible participants will be required to provide informed consent (online supplemental material 1) themselves or through a family member.
Supplemental material
Randomisation and blinding
20 care facilities will be randomly selected from among the care facilities operated by the HKSKH. The level of care and resident characteristics are similar across these facilities. The 20 facilities will be randomly allocated to either the intervention group (receiving Mobility-Fit) or the control group (receiving a lower limb strengthening programme as part of the standard care), in a 1:1 ratio without restriction (with 80 participants in each group). We use a cluster RCT because randomisation at the cluster (ie, care facility) level will reduce the likelihood of experimental contamination. Random allocation will be conducted by an independent statistician using a computer-generated minimisation programme after the baseline assessment of the participants. The randomisation will be stratified according to care facility in order to have both groups distributed as equally as possible in each care facility. The research assistants who conduct the assessments will be blinded to the randomisation and allocation of participants. Due to the nature of this study, it will not be possible to blind the group allocation from the instructors involved in the training of the intervention group. Participants (or their families) will not be informed regarding to which group they belong. A timeline for recruitment, allocation, intervention and assessment of the participants is presented in table 2.
Schedule of participant enrolment, interventions and assessments
Intervention
Physiotherapists (PTs) and recreation workers at the care facilities will be the programme instructors for both the intervention and control groups. Prior to delivery of the Mobility-Fit programme, the instructors will meet with our research team to review the study protocol. The instructors will receive training (in-person and online) of the Mobility-Fit programme by our team. After the training, instructors will review the baseline measures (described below) of the participants to understand their physical and cognitive capacities. The instructors will set a goal with each participant at the beginning of the 12-week programme and then customise and adjust the programme intensity based on the participant’s level of functioning and progress. During programme delivery, a well-trained research assistant (who is not involved in assessments) will facilitate the exercise sessions. To measure participant adherence, the recreation worker and well-trained research assistant will document the attendance of each participant and how many of the exercises (eg, components of the programme) are completed by the participant in each session. During the 9-month follow-up period after the intervention, the PTs and recreation workers at the care facilities will continue to deliver the Mobility-Fit programme. The well-trained research assistant will visit the facilities weekly during the follow-up to maintain regular communication with staff and participants.
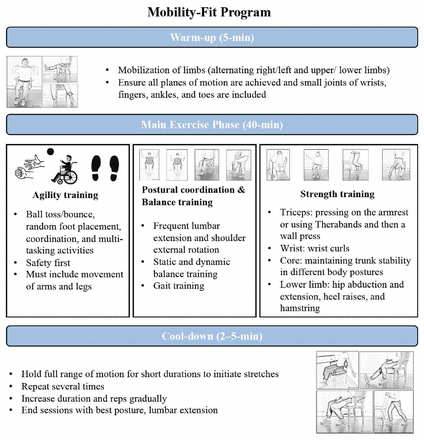
The Mobility-Fit programme was designed for older adults residing in care facilities16 to enhance their ADL performance. The session commences with a 5 min warm-up that includes joint range of motion exercises in all planes of movement. After the warm-up, the participants begin seated exercises and then progress to standing exercises (they can hold a chair if needed). The 40 min main workout includes agility, postural coordination, balance and strength training (triceps, wrist, core and lower limb strength) (figure 2). Participants with mobility issues (eg, using a wheelchair or walker) can practice while holding/leaning on a chair or wheelchair.16 The selection of exercises and activities is based on the participants’ mobility ability, strength, stamina and cognitive function. Advancement is encouraged as individuals achieve exercises with proper posture and technique. For safety considerations, standing activities are chosen and sturdy support is provided. It is essential to maintain a realistic approach as not all participants may reach the highest levels of progression. Our previous literature review suggested a dose–response relationship with a 12-week programme, consisting of three sessions per week, in improving mobility in the residential care setting.14 Consequently, participants in the intervention group will attend the Mobility-Fit programme led by a facility-based instructor three times per week, 45 min per session for 12 weeks. The control group will participate in an existing exercise programme (part of the standard care) offered by the care facility for 12 weeks. The programme consists of lower limb stretching, quadriceps strengthening in a sitting position and strengthening of hip extensors and abductors in a standing position. The standard care programme will also be held three times per week, with each session spanning 45 min.
Schematic of the Mobility-Fit programme.
Data collection and outcome measures
Data will be collected at each care facility. Demographic information (age and gender), height and weight, fall history and chronic disease diagnoses of the participants will be acquired from the Minimum Data Set (MDS) or other pertinent health records. At least two well-trained research assistants will perform the outcome measures with the participants at baseline, after completion of the intervention and after completion of the follow-up period (table 2). During the tests, one assistant will stand beside the participant for safety. After the intervention, 30 randomly selected participants (3 per intervention cluster) and their family members and 20 care staff members (2 per intervention cluster), including exercise instructors, managers and recreation workers, will be interviewed to evaluate the fidelity, sustained effects, programme delivery barriers and facilitators, and health economics of the Mobility-Fit.
For objectives 1 and 2
Primary outcomes
Upper limb strength: (1) the Hoggan microFET V.2 hand-held dynamometry (HHD) (Hoggan Scientific, Salt Lake City, Utah, USA) will be used to evaluate the triceps and biceps strength.23 The participant will place the elbow flexed at 90° on the table, and the HHD will be held on the inside and outside of the distal forearm of the participant, and then the participant will be instructed to press against the HHD and hold it for 3 s and (2) handgrip strength will be measured using an HHD (5001 Grip-A; Takei, Niigata City, Japan).24 The upper limb being tested will naturally hang down and the participant will be asked to grip the dynamometer handle as firmly as possible for 3 s, preferably in a standing position. Testing will be performed on the participant’s dominant side. Two trials of each test will be conducted and the highest value will be used for data analysis.
Lower limb strength: quadriceps strength will also be assessed using the Hoggan microFET V.2 HHD.25 Participants will be seated in a chair, with the chair height adjusted to ensure their feet are off the ground. The assessor will place the HHD on the anterior aspect of the dominant side’s distal tibia. Participant will be instructed to exert force against the HHD in the anterior aspect of the thigh and hold it for 3 s. Three trials of each test will be conducted and the highest value will be used for data analysis.
Trunk stability: postural sway will be measured in accordance with the Physiological Profile Assessment (PPA).26 Participant will be asked to stand with their eyes open for 30 s and a sway metre will be secured at waist level. A research assistant will stand beside the participant to ensure safety throughput the test. The total sway and anteroposterior and mediolateral sway will be recorded. Two trials will be conducted and the mean will be used for data analysis.
Reaction time: reaction time will also be assessed using the PPA.26 Participants will be allowed to perform 5 practice trials followed by 10 formal experimental trials, and the mean will be calculated for data analysis.
Mobility function: we will use the Short Physical Performance Battery (SPPB) to measure mobility function.27 The SPPB includes walking 4 m, time to complete sit-to-stand five times and standing balance in different positions (side by side, semitandem and tandem).
Fall efficacy: this will be measured using the Fall Efficacy Scale International.28
Secondary outcomes
Daily PA level and performance: following the direct assessments, the participants will wear a sensor on their right waist to continuously monitor their daytime PA levels. We will use a triaxial accelerometer (±16G) and gyroscope (±2000°/s) device (ActiGraph GT9X Link IMU, Pensacola, Florida, USA) to capture their movement patterns at a sampling frequency of 100 Hz. Four days are considered sufficient to obtain reliable estimates of daily PA.29 Thus, each participant will be monitored for approximately 10 hours during the daytime for five consecutive days. Specifically, the research assistants will go to the care facilities in the morning to put the sensor on the participant’s waist. They will also observe the participants in common areas during the day and document activities that are not captured by the sensors (eg, walking in hallways or watching TV). The sensors will be taken off in the evening and securely stored at the facility. This testing protocol has been previously employed in our pilot study16 and showed 100% adherence and reliability in measuring PA. For outcomes related to movement quantity (PA level), we will use average activity counts per day,3 30 upright time29 and sitting time during the day. Sedentary time will also be calculated. Data processing will be carried out using MATLAB. Our activity classification algorithm will be based on accelerometers and gyroscope data from each axis (X, Y, Z) using the following features: mean, variance, mean SD, IQR, correlation between axes, kurtosis, number of zero crossings and fast Fourier transform energy.31 32 For outcomes related to movement quality (performance), we will measure gait speed during walking, trunk kinematics during transferring and sway during standing.32 33
Frailty: we will assess frailty using the 7-item FRAIL-Nursing Home (FRAIL-NH) Scale, which comprises fatigue, resistance, mobility, incontinence or disease, weight loss, eating style and assistance with dressing (all items will be derived from the MDS). Each item is worth 0, 1 or 2 points, with a total score of 14. The scale’s scoring categorises participants as either robust (score of 0), pre-frail (score 1–4) or frail (score≥5). This tool has been previously used in Hong Kong.19
Cognitive function: the Hong Kong version of the Montreal Cognitive Assessment (HK-MoCA)34 will be employed to evaluate the cognitive function of participants. The HK-MoCA is a free of charge and easy to administer tool that has been validated for use in the Hong Kong population.
Quality of life: health-related quality of life will be measured using the EuroQol- 5 Dimension (EQ-5D) (from EuroQol, available in Chinese). The EQ-5D provides utility weights that will allow calculation of quality-adjusted life years (QALYs) for use in the economic evaluation35 in objective 3.
For objective 3
Adherence and fidelity to the programme: we will document the attendance and the percentage of completed exercises per session for participants enrolled in the Mobility-Fit programme.
Goal attainment: in the intervention group, we will assess goal attainment using a 4-point scale: deteriorated from baseline ability, maintained baseline ability, goal met or goal exceeded at intervention completion.
Adverse events: these include medical events or injuries resulting from the interventions that limit medical care or restricted ADLs for more than 2 days. Adverse events will be monitored during the intervention and 9-month follow-up.
Interviews regarding programme delivery: semistructured interviews will be conducted with participants (n=30), their family members and caregivers (n=20) at intervention completion and at the end of the follow-up period. Interview questions will be guided by a protocol developed by the research team and stakeholders. Specifically, these interviews will aim to elicit information on the perceived benefits of the programme, interest in the programme, whether type and intensity of the exercises were appropriate and any changes in daily PAs after the programme. We will also inquire about specifics related to programme delivery, such as the clarity of instructions, peer and staff support, and the format of the programme (eg, practising at one’s own pace with progression). The interviews will consist of open-ended questions and narrative responses. To ensure a well-rounded understanding of our programme delivery, an independent statistician will employ stratified sampling to select a mixture of older adults, including those who have benefitted the most, the least (based on outcome changes), and have dropped out from the intervention. During the intervention, a well-trained research assistant will conduct the interview while another will record field notes along with audio recording. Both research assistants will be blinded to the participants’ allocation.
Fall incidence, hospitalisations and admissions to palliative care: this information will be collected via telephone and incident reports at intervention completion and at the end of follow-up. This information will be used in the economic analysis.
Health and community service use: this information will be documented and confirmed with the care facilities at intervention completion and at the end of follow-up. It will also be used in the economic analysis.
Data analyses
For objective 1
SPSS (V.26) will be used for the statistical analyses. We will employ intention-to-treat analysis36 whereby participants will be analysed in the group to which they were allocated. Baseline characteristics will be compared with adjustment for any identified systematic group differences. Outcome variables will be compared over time and between groups. For repeated measurements, the outcome variables will be correlated over time for each participant. To avoid incorrect inferences due to underestimated standard errors and inefficient estimators (ie, more mean square error in regression parameter estimators than necessary), we will apply generalised linear mixed models (GLMMs) for continuous outcome variables. To further enhance the statistical efficiency, we will consider various correlation structures (eg, unstructured, compound symmetry) to determine the best correlation structure for our dataset. For discrete outcome variables (eg, binary and count data), we will apply generalised estimating equations (GEEs). Covariates will include age, gender, HK-MoCA scores, the use of mobility aids and diagnosed conditions. All statistical tests will be two tailed, with statistical significance set at p<0.05. Bonferroni adjustment will be used in the presence of multiple significance tests. We will use GLMM and GEE models because they have the ability to incorporate individual-level covariates in cluster-level analyses.37 They also involve intracluster correlation in the modelling process, resulting in a more realistic representation of the clustered data. Furthermore, GLMM and GEE can handle missing data by using maximum likelihood estimation, ensuring an unbiased estimation.
For objective 2
We will examine the programme’s effects on subgroups of older adults. For frailty, participants will be categorised into two subgroups based on their FRAIL-NH Score at baseline: a non-frail and pre-frail group (scores 0–4) and a frail group (scores≥5). The mobility subgroup will be determined using baseline SPPB scores, with individuals categorised into a higher fall risk group (scores<9) and a lower fall risk group (scores≥9). The cognition subgroups will be based on HK-MoCA scores at baseline, and the cut-off has been validated in the Hong Kong population34: a normal cognition group (scores≥22) and a cognitive impairment group (scores≤21). The independent variables will include the activity allocation (intervention or control) and one of these defined subgroups for each analysis. Potential interactions between activity allocation and each subgroup will be explored using repeated measures ANOVA for continuous outcome variables and logistic regression for categorical variables. On completion of the intervention, we will proceed to analyse between-group differences in the outcome variables as outlined in objective 1. Throughout these analyses, age and gender will be incorporated as covariates to account for potential confounding factors.
For objective 3
For adherence and intervention fidelity, we will employ descriptive statistics to report the attendance rate of participants in all sessions of the Mobility-Fit programme. Additionally, we will provide information on the percentage of exercises completed by the participants during the intervention sessions. Goal attainment will be described in terms of the percentage of participants in each category. As for adverse events, we will also report these using descriptive statistics, offering a clear and concise account of any incidents that may have occurred during the course of the study.
For sustained effects, we will employ the same analytical approaches used in objective one for the quantitative data (eg, strength and mobility outcomes and sensor data). In the repeated measures analyses, the independent variable ‘time’ will include baseline, completion the intervention and follow-up assessments, to examine whether mobility function and physical performance were maintained over the follow-up period.
Regarding the evaluation of programme delivery, we will conduct a thematic analysis38 to identify thematic patterns from the interview data related to the barriers and facilitators to programme implementation. The interview data will be managed using a qualitative data management software (Nvivo V.12), and the team members responsible for the analysis will be blinded to the randomisation of participants. Our analysis process will adhere to the six steps outlined by Braun and Clarke38: (1) Transcribing the interview data by listening to audio recordings multiple times to allow familiarisation with the content. (2) Importing the transcripts into Nvivo for initial coding to identify particular features of the dataset. (3) Organising the preliminary codes into potential themes. Team members will review the codes associated with initial themes and then discuss whether any adjustments are necessary. (4) Developing a thematic map with the coded data considering the alignment of themes and subthemes. (5) Deriving clear and distinct definitions for each identified theme. (6) Using the defined themes to describe outcomes (eg, barriers, facilitators) to address the research objectives.
We will carry out the economic evaluation and generate reports according to health economics reporting standards.39 The economic evaluation will adopt the perspective of Hong Kong’s elderly care service providers over a 12-month period. Benefits will be measured in terms of the number of transitions to frailty prevented, mobility improvement and QALYs gained (based on utility weights derived from EQ-5D). The cost-effectiveness analysis will include the costs of delivering the intervention, as well as the costs of health and community service utilisation. Bootstrap sampling will be used to examine the joint probability distribution of costs and outcomes, with the creation of incremental cost-effectiveness planes and cost-effectiveness acceptability curves for each outcome in the analysis.
Patient and public involvement
None.
Ethics and dissemination
This study will be conducted in accordance with the World Medical Association Declaration of Helsinki. Ethics approval has been acquired from the Research Ethics Board at the Chinese University of Hong Kong and the Joint CUHK-NTEC Clinical Research Ethics Committee (SBRE-21-0413 and CREC-2022-459). Informed consent will be obtained from participants. We consider this study to have a low risk to participant safety. The Mobility-fit programme involves multiple components relevant to the performance of daily activities in older adults. Participants in the intervention group will start from basic activities during sitting and then progress to exercises during standing. Our programme has been tested for feasibility in frail older adults and no adverse event occurred. The programme will be delivered by facility-based PTs and their assistants. They will closely monitor the progress and issues of the participants. Therefore, the risk of musculoskeletal complaints and accidental falls should be low. We expect that our PA programme would provide more benefit than harm when practised in reasonable amounts, which will be closely guided by the in-house PTs.
The study findings will be disseminated via peer-reviewed journals, national and international conference presentations and social media. For secondary analysis of the data, please contact the corresponding author. The results of this study are expected to provide evidence regarding a comprehensive and effective multicomponent PA programme on fall prevention for older adults living in care facilities. The Mobility-Fit programme comprises agility, postural coordination, balance and strength training (triceps, wrist, core and lower limb strength), with suitable dosage based on participant’s physical and cognitive function. The cost-effectiveness evaluation will be a critical step in convincing healthcare practitioners and public health policymakers to widely implement Mobility-Fit programme to tailor intervention strategies for older adults living in care facilities as part of routine healthcare.
Ethics statements
Patient consent for publication
Acknowledgments
We thank the Hong Kong Sheng Kung Hui (HKSKH) Multi-disciplinary Outreaching Support Team for the Elderly for their partnership in our research. We also thank Mr Francis Chan, Mr Chris Fung and other care staff who dedicated time to the recruitment and promotion of the Mobility-Fit programme.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @Sum Kim Wai Raymond
Contributors YY conceived the idea for the trial and oversaw the development of the Mobility-Fit programme, assisted by KSvS, RS and DC. The trial was designed by YY, assisted by KSvS, RS and implemented by YY, DC, ZZ, JS, C-yH and K-pC. Funding was obtained by YY, and YY and ZZ prepared the first draft of the manuscript. All authors contributed to the refinement of the study protocol and approved the final version of the manuscript.
Funding This work was supported by the Research Grants Council of Hong Kong (RGC-HK, grant number: CUHK 24618722). The funding source had no role in the design, methods, subject recruitment, data collections, analysis and preparation of this article.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.