Article Text
Abstract
Objective Cardiac CT (CCT) is an emerging non-invasive modality for assessing left atrial appendage (LAA) thrombus, but the results were conflicting. Our study aims to evaluate the accuracy of CCT for detecting LAA thrombus in patients undergoing catheter ablation of atrial fibrillation, using trans-oesophageal echocardiography (TEE) as the reference standard.
Design Case-control study.
Setting Patient data were collected from a tertiary hospital in China between 2017 and 2022.
Participants The study enrolled 726 patients (male: 60.2%, age: 61±11 years) who had both TEE and CCT before catheter ablation of atrial fibrillation.
Measures The CCT protocol consisted of one angiographic phase and one delayed scan 30 s later. LAA thrombi were defined as solid masses on TEE or persistent defects on CCT. The thrombus dimension and location, the LAA filling and emptying flow velocity were assessed by TEE.
Results Of the 57 (7.9%) patients with LAA thrombi identified by TEE, 29 (50.9%) were located at the LAA ostium, and 28 (49.1%) were in the LAA. The former showed higher motility following blood flow and heartbeats than the latter. The CCT detected 14 (48.3%) of the LAA-ostium thrombi but 25 (89.3%) of those in the LAA (p=0.001). The LAA-ostium thrombi with the LAA mean flow velocity >0.35 m/s and maximum diameters <10 mm were more prone to have CCT false-negative results.
Conclusion For patients undergoing catheter ablation for atrial fibrillation, CCT with a 30 s delay scan is less sensitive to LAA thrombi than TEE, especially for LAA-ostium thrombi with smaller sizes and higher LAA flow velocity.
- stroke
- ultrasound
- computed tomography
- thromboembolism
Data availability statement
Data are available on reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
A major strength of our study is based on a large cohort with high incidence of left atrial appendage thrombi.
We first report the impact of thrombus location on the sensitivity of cardiac CT (CCT), using trans-oesophageal echocardiography as reference standard.
A limitation of this study is that it is a single-centre and retrospective study.
The CCT protocol contained only one delayed-phase scan.
Whether multiple delayed-phase scans reduce false-negative CCT results is unclear.
Introduction
Trans-oesophageal echocardiology (TEE) is currently the gold standard for the assessment of left atrial appendage (LAA) thrombi in patients undergoing catheter ablation of atrial fibrillation (AF).1 In the past decades, cardiac CT (CCT) has been widely studied for detecting LAA thrombus.2–5 Recently, the delayed contrast-enhanced CCT at 6 min was reported to reach 100% sensitivity and negative predictive value in detecting LAA thrombus.6 For patients who are not tolerant of the discomfort associated with TEE, CCT is proposed as a reliable alternative.7 However, the accuracy of CCT varied between studies,2–5 8 raising the safety concern and limiting the routine use of CCT.
The left atrial thrombi were classified into a movable ball, fixed ball and mountain according to their morphology.9 Around one-third of the thrombi in the left atrium were movable. Although CCT is of high spatial resolution, its temporal resolution is 4~17 Hz, and it takes 60~250 ms to acquire one slice.8 10–12 Compared with TEE, which allows live display with a high spatiotemporal resolution (<1 mm, 20~30 Hz),13 CCT is theoretically inferior for identifying moving objects in the left atrium. In addition, the circulatory stasis and the fibrillation of the atrial wall during AF also increase the difficulty of detecting movable thrombi.14 In previous studies, the presence of LAA thrombus was 2 (0.24%)~16 (19.04%).2–5 15 The high sensitivity and negative predictive value of CCT could be attributed to the low incidence of movable thrombus. Therefore, it is essential to investigate the diagnostic accuracy of CCT in a larger cohort with a higher incidence of LAA thrombus.
In the current study, we retrospectively included 726 patients who had both TEE and CCT before catheter ablation for AF. 57 (7.9%) patients were diagnosed with LAA thrombi using TEE. We aimed to evaluate the sensitivity of CCT in detecting LAA thrombus and to analyse the risk factors of false-negative results.
Methods
Study population
From May 2017 to December 2022, patients were routinely scheduled for TEE and CCT before catheter ablation of AF in Guizhou Provincial People’s Hospital. Those who had both examinations within a 12-hour interval on the same day were retrospectively included. All the patient characteristics and images were acquired from the clinical database. The CHA2DS2-VASc score was calculated following the 2020 European Society of Cardiology (ESC) guideline.1
CCT protocol
All patients were examined with a third-generation dual-source CT (SOMATOM Force, Siemens Healthineers, Forchheim, Germany). The imaging protocol included a standard angiographic-phase acquisition to assess the anatomy of the left atrium and one delayed-phase acquisition at 30 s after contrast injection to detect thrombi. No electrocardiography gating was used. The tube voltage and current were automatically adjusted using the CARE kV or CARE DOSE 4D with the reference of 100 kV and 250 mA. The gantry rotation time was 250 ms, and the detector collimator was 192×0.6 mm. The scanning image covers the range from the aortic arch to the cardiac base. The monitoring site was localised within the left atrium. The contrast material containing 350 mg/mL of iodine was administrated intravenously at 4–5 mL/s with a total dose of 35 mL by a double-barrel syringe, followed by the same amount of 0.9% saline. For the angiographic phase, the scanning was triggered when the monitoring site reached 90 HU. Then the delayed phase was acquired 30 s later. All images were reconstructed using the ADMIRE algorithm grade 3 with a layer thickness of 1 mm and interlayer spacing of 0.7 mm. The three-dimensional (3D) left atrium and pulmonary veins were reconstructed using the Syngo.via software (Siemens Healthcare, Forchheim, Germany).
CCT image analysis
Images were transferred to an external workstation (Syngo VPCT body, Siemens Healthcare). Two independent radiologists with >8 years of cardiovascular imaging, blinded to all patients’ data, retrospectively evaluated the presence of LAA thrombus and LAA morphology. Thrombus was defined as a persistent filling defect in the 30 s delayed scans. The LAA morphology was classified into simple or complex according to their main and branching structures on the 3D reconstructions.16 In case of disagreement, a final joint reading was performed to achieve consensus.
Trans-oesophageal echocardiology
A PhilipsiEPIQ7C colour Doppler ultrasound diagnostic instrument (Philips, Amsterdam, the Netherlands) and an X7-2t probe were used. The left atrium and LAA were scanned using a complete two-dimensional (2D), coloured, pulsed and continuous-wave Doppler echocardiogram according to the European Association of Cardiovascular Imaging recommendations.17 Thrombi were defined as circumscribed echogenic or echolucent masses distinct from the atrial wall. LAA ostium was defined as within the 5 mm range of the junction between the smooth atrial wall and trabeculated LAA. The dimensions and locations of thrombi were assessed if present. The LAA filling and emptying velocity was averaged by 10 consecutive measurements of the backward and forward flow rates using the pulsed wave Doppler. LAA mean flow velocity (LAAMFV) was calculated by halving the sum of the LAA filling and emptying velocity. Two independent cardiologists with >5 years of experience in echocardiology evaluated the presence of LAA thrombus and measured the flow velocity. Disagreements were resolved by consensus.
Statistical analysis
Continuous variables were expressed as mean±SD. The Student’s t-test was used for comparison if normally distributed; otherwise, the Mann-Whitney U test was used. Categorical variables were expressed as frequency (percentage). The χ2 test was used for comparison if the expected frequency was >5; otherwise, Fisher’s exact test was used. A two-tailed p value of <0.05 was considered statistically significant. Using TEE as the reference standard, the diagnostic performance of CCT for LAA thrombi was calculated. Multivariate logistic regression model was conducted to determine the predictors of CCT false-negative results. The receiver operating characteristics (ROC) curve with the area under the curve (AUC) was calculated to evaluate the performance of the predictive model. All statistical analysis was performed using SPSS V.26.0.
Results
Patient characteristics
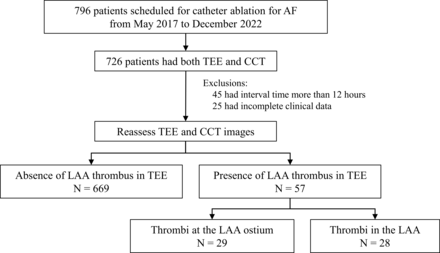
A total of 726 patients (age: 61±11 years; male gender: 60.2%) with 57 (7.9%) LAA thrombi diagnosed by TEE were included in the study (figure 1). The patients with LAA thrombus had a higher incidence of previous stroke/transient ischaemic attack (TIA) and congestive heart failure, larger left atrial size, worse left ventricular function and a higher rate of complex LAA morphology than those without LAA thrombus (p<0.05) (table 1).
Patient flow chart. AF, atrial fibrillation; CCT, cardiac CT; LAA, left atrial appendage; TEE, trans-oesophageal echocardiography.
Patient characteristics
Twenty-nine (50.9%) of the thrombi were located at the LAA ostium and 28 (49.1%) were in the LAA. The clinical characteristics and maximum thrombus diameter were similar between the patients. The thrombi at the LAA ostium were accompanied by higher LAA filling and emptying velocities than those in the LAA (online supplemental table S1). The former also showed significantly higher mobility following the blood flow and cardiac motion than the latter (online supplemental video S1).
Supplemental material
Supplementary video
Diagnostic accuracy of CCT
The CCT had an overall sensitivity of 68.4% and a specificity of 99.4% to the LAA thrombi (table 1). However, the CCT was less sensitive to the thrombi at LAA ostium than those in the LAA (48.3% vs 89.3%, p=0.001) (online supplemental table S1). Representative images are shown in figure 2.
Representative CCT and TEE images of the LAA thrombi. The left and middle images are from the CCT angiographic and delayed phase. The right images are from the TEE. (A–C) Representative false-negative CCT result of the thrombus in the LAA. (D–F) Representative false-negative CCT result of the LAA-ostium thrombus. (G–I) Representative true-positive CCT result of the thrombus in the LAA. (J–L) Representative true-positive CCT result of the LAA-ostium thrombus. White arrows denote the thrombi. CCT, cardiac CT; LAA, left atrial appendage; TEE, trans-oesophageal echocardiography.
Given that a high sensitivity is essential to exclude LAA thrombus, the CCT results of the LAA thrombi underwent further analysis. Compared with the CCT true-positive thrombi, the false-negative ones more frequently located at the LAA ostium (83.3% vs 35.9%, p=0.001), were shorter in the maximum diameters (8±2 mm vs 14±9 mm, p<0.001) and were accompanied by higher LAA filling and emptying velocities (0.53±0.15 m/s vs 0.38±0.19 m/s, p=0.001; 0.55±0.21 m/s vs 0.37±0.14 m/s, p<0.001) (table 2). The result was also concordant in the LAA-ostium thrombi subgroup (online supplemental table S2). By scattering the CCT results on the 2D plane that consisted of LAAMFV and thrombus maximum diameter, the thrombi at the LAA ostium with the LAAMFVs higher than 0.35 m/s and maximum diameters <10 mm were more prone to have CCT false-negative results (figure 3A). For the thrombi in the LAA, the maximum diameter or LAAMFV did not impact the CCT results (figure 3B). In the logistic regression model, the thrombus maximum diameter, location and LAAMFV were independently associated with the CCT false-negative results (online supplemental table S3). The ROC curve (AUC 0.909, 95% CI 0.834 to 0.984) is shown in online supplemental figure S1.
Supplemental material
Supplemental material
Supplemental material
Scatter plots of CCT results. Red dots denote the CCT false-negative thrombi. Green dots denote the CCT true-positive thrombi. The LAAMFV was calculated by halving the sum of the LAA emptying and filling velocity. (A) For the LAA-ostium thrombi, the CCT false-negative results had the LAAMFV >0.35 m/s and maximum diameters <10 mm. (B) For the thrombi in the LAA, the maximum diameter or LAAMFV did not significantly impact CCT results. CCT, cardiac CT; LAA, left atrial appendage; LAAMFV, left atrial appendage mean flow velocity.
Patient characteristics between CCT true-positive and false-negative results
Discussion
Major findings
We re-evaluated the accuracy of CCT for detecting LAA thrombus in the 726 patients undergoing catheter ablation for AF. TEE identified 57 (7.9%) patients with LAA thrombi, of which 29 (50.9%) were at the LAA ostium and 28 (49.1%) in the LAA. The CCT was less sensitive in detecting LAA-ostium thrombi with smaller maximum diameters and higher LAAMFV.
Concordance
LAA thrombus is thought to form initially inside the LAA, where circulatory stasis is the greatest, and then extends towards the ostium. However, we found over half of the thrombi located at the LAA ostium. The result was concordant with a previous study in which 46.3% of the thrombi were at the entrance section of LAA.9 The presence of pits and troughs adjacent to the LAA orifice was reported in 57.7% of the human heart specimens.18 It could be the anatomic basis for thrombosis. Another study using TEE discovered small recesses proximal to the entry of LAA in a significant number of patients, but no thrombus related to these structures was detected following LAA occlusion.19 The discrepancy could be due to patient selection bias. Nonetheless, our findings raised concerns about the role of LAA-ostium recesses in thrombosis.
Although the third-generation 64-slice dual-source CT was employed in the current study, its sensitivity to LAA thrombi was not improved. The sensitivity of 89.3% to the thrombi in the LAA was comparable to the previous studies.2 10 11 20–22 However, the sensitivity significantly decreased for the LAA-ostium thrombi, which showed higher motility following blood flow and heartbeats than those in the LAA (online supplemental video S1). We further identified that the LAA-ostium thrombi with smaller sizes and higher LAA flow velocity were more prone to be missed using the CCT. As a higher temporal resolution is essential to detect movable objects,23 our findings suggested the impact of thrombus motility on CCT imaging. By calculating the gantry rotation time of 250 ms, the CCT had a temporal resolution of 4 Hz, significantly less than that of the TEE (20~40 Hz). Thus, the low sensitivity of CCT in our study could be presumed to: (1) inadequate temporal resolution, (2) the high proportion of movable LAA-ostium thrombi, (3) the lack of ECG gating, (4) only one 30 s delayed scan was used. The four CCT false-positive results in our study could be the misdiagnosis of thrombus due to circulatory stasis during AF.6 24
Discordance
Several studies reported that the CCT had 100% sensitivity in detecting LAA thrombi using ECG gating, single-segment reconstruction algorithm25 26 or multiple delayed-phase scans.3 14 27 However, these studies have the following limitations: (1) although the single-segment reconstruction algorithm increased the CCT’s temporal resolution to 12 Hz, the low incidence of LAA thrombus (2 (0.24%)~16 (19.04%)) and the less variation of thrombus location could have overestimated the sensitivity of CCT for detecting LAA thrombi in a broader range of patients; (2) the multiple delayed-phase scans distinguished the thrombi from filling defects due to circulatory stasis. Still, the technique could not necessarily increase the sensitivity to smaller and movable thrombi at the LAA ostium.
We suggest that the CCT approach to detect LAA thrombi could differ according to their location and motility. Persistent filling defects in multiple delayed-phase scans have been well established for static thrombi in LAA. However, LAA-ostium thrombi could be highly motile due to blood flow and myocardial contraction. In our study, the LAA flow velocity indirectly reflected the thrombus motility. The filling defects that indicate the presence of thrombi could still be enhanced as the thrombi swayed much faster than the duration required for scanning. Furthermore, the filling defect size also depended on the thrombi size. Therefore, smaller LAA-ostium thrombi with higher LAA flow velocity could be prone to be missed by CCT. A temporal resolution comparable to TEE (20~40 Hz) could be necessary.
Clinical implication
Our study found that the CCT with a 30 s delayed scan did not reliably exclude LAA thrombus, especially those located at the LAA ostium. We did not find any clinical characteristics associated with the thrombus location. TEE is the only recommendation for stroke risk management for patients undergoing catheter ablation of AF in the 2020 ESC guideline.1 For those who do not tolerate TEE, intracardiac echocardiography could be considered.28
Limitations
First, this retrospective single-centre study included only patients undergoing catheter ablation for AF. The study’s findings may not be generalisable to other patients with AF. Second, the CCT protocol contained only one delayed-phase scan. Whether multiple delayed-phase scans reduce false-negative CCT results is unclear. Third, other factors unaccounted for in this analysis—heart rhythm, atrial/ventricular rate and blood pressure during the TEE and CCT—could affect the conclusions. Fourth, although the interval between the TEE and CCT was <12 hours, it is theoretically possible for thrombi to form or disappear within this timeframe. Lastly, the incidence of LAA thrombus was higher than that in previous studies (partially due to less adherence to anticoagulation treatment), which could have affected the thrombus location and motility.
Conclusions
In patients undergoing catheter ablation of AF, CCT with a 30 s delay scan is less sensitive than TEE in detecting LAA thrombi, especially those located at LAA ostium with smaller sizes and higher LAA flow velocity.
Data availability statement
Data are available on reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
The study was approved by the institutional ethics committee of Guizhou Provincial People’s Hospital. The informed consent was waived due to the retrospective nature, and no identified information was collected.
Acknowledgments
We thank Nicholas Tan and Jason Tri from the Mayo Clinic for their dedicated help in preparing the manuscript.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
CG and ZJ contributed equally.
Contributors CG and ZJ: conceptualisation, methodology, formal analysis, writing—original draft, writing—review and editing. JHe, HM and YW: conceptualisation, methodology, formal analysis, visualisation, writing—review and editing. JT: conceptualisation, methodology, investigation, writing—review and editing. QO: conceptualisation, investigation, writing—review and editing. YT and QL: conceptualisation, writing—review and editing, funding acquisition. LT and JHu: conceptualisation, investigation, writing—review and editing. LY: conceptualisation, methodology, formal analysis, writing—review and editing, supervision, funding acquisition. LY is the guarantor of the study. All authors read and approved the final manuscript.
Funding This study has received funding from the Science and Technology Support plan of Guizhou Province (no. (2017) 2885 and (2018) 2794) to LY and YT, the Clinical Special Projects in Guizhou Province (no. (2019) 4430) to QL and the Clinical Research Center Project of the Department of Science and Technology of Guizhou Province (no. (2017) 5405) to LY.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.