Article Text
Abstract
Introduction Current treatments for pain in breast cancer survivors (BCSs) are mostly biomedically focused rather than biopsychosocially driven. However, 22% of BCSs with pain are experiencing perceived injustice, which is a known predictor for adverse pain outcomes and opioid prescription due to increased maladaptive pain behaviour. Educational interventions such as pain neuroscience education (PNE) are suggested to target perceived injustice. In addition, motivational interviewing can be an effective behavioural change technique. This trial aims to examine whether perceived injustice-targeted PNE with the integration of motivational interviewing is superior to biomedically focused pain education in reducing pain after 12 months in BCS with perceived injustice and pain. In addition, improvements in quality of life, perceived injustice and opioid use are evaluated, and a cost-effectiveness analysis will finally result in a recommendation concerning the use of perceived injustice-targeted PNE in BCSs with perceived injustice and pain.
Methods and analysis This two-arm multicentre randomised controlled trial will recruit female BCS (n=156) with pain and perceived injustice. Participants will be randomly assigned to perceived injustice-targeted PNE or biomedically focused pain education in each centre. Both interventions include an online session, an information leaflet and three one-to-one sessions. The primary outcome (pain), secondary outcomes (quality of life, perceived injustice and outcomes for cost-effectiveness analysis) and explanatory outcomes (pain phenotyping, sleep, fatigue and cognitive-emotional factors) will be assessed at baseline and at 0, 6, 12 and 24 months postintervention using self-reported questionnaires online. Treatment effects over time will be evaluated using linear mixed model analyses. Additionally, a cost-utility analysis will be done from a healthcare payer and societal perspective.
Ethics and dissemination The ethical agreement was obtained from the Main Ethics Committee (B.U.N.1432020000068) at the University Hospital Brussels and all other participating hospitals. Study results will be disseminated through presentations, conferences, social media, press and journals.
Trial registration number NCT04730154.
- Breast tumours
- Cancer pain
- Pain management
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This trial is the first to examine the (cost-)effectiveness of an innovative perceived injustice-targeted intervention in the cancer population.
A multicentre study including both university and peripheral hospitals.
Comparison of balanced treatment arms.
A blended intervention with patient-centred disease management.
Due to the innovative nature of this study, results will not be easily compared with other study trials.
Introduction
Worldwide, the most frequently diagnosed malignancy in women is breast cancer.1 The Global Burden of Disease Cancer Collaboration demonstrated that 2.3 million people were diagnosed with breast cancer in 2020, accounting for 11.7% of all cancer cases.2 3 Due to improved screening and treatment methods, survival rates have increased to 90.0% 5 years after diagnosis in high-income countries.4 5 Since the number of survivors is increasing, new long-term symptoms are emerging after treatment.6 After fatigue, pain is the most frequent treatment-related debilitating morbidity.7 The prevalence of persistent pain is 29.8% post surgery, 27.3% postradiotherapy and 21.8% after receiving various combinations of breast cancer treatment.8 This high prevalence is of considerable concern since pain not only impacts the quality of life but also prevents the reuptake of activities, leading to a huge socioeconomic burden.9–13 In 22% of breast cancer survivors (BCSs) with pain, feelings of injustice are reported.14
Perceived injustice has been conceptualised as a multidimensional appraisal process characterised by a tendency to interpret one’s losses as severe and irreparable, to attribute blame to others for one’s suffering and to experience a sense of unfairness15 (eg, someone who survived breast cancer but has to deal with pain afterwards). Perceived injustice inherently presumes a discrepancy between expected and actual outcomes, which may lead to feelings of anger, frustration or other forms of emotional distress.16 Perceived injustice is also associated with increased opioid prescription17 and predicts opioid use at 1 year,18 urging the need for targeted interventions diminishing perceived injustice. Individuals who view their pain as unjust may display more pain behaviour by an intense communication of their suffering and losses increasing the likelihood of being prescribed opioids.17
Perceived injustice is related to lower quality of life, and perceived injustice rather than pain catastrophising mediates the relationship between pain and quality of life in BCSs.14 Moreover, perceived injustice is an important mediator in the relationship of pain on fatigue and sleep.19 The mediating effect of perceived injustice with quality of life, sleep and fatigue among BCSs shows that perceived injustice is not only understudied but also underappreciated and undertreated.20 Therefore, it might be important to incorporate perceived injustice as a treatment target in the rehabilitation of BCSs. Literature suggests the use of cognitive-behaviour interventions, pain acceptance15 and educational interventions comprising elements of reassurance and encouragement towards activity re-engagement.21 One such intervention is pain neuroscience education (PNE).
PNE is an intervention primarily aiming at changing illness perceptions of patients with pain. During this education, easy-to-understand pain neurophysiology examples (central sensitisation) and metaphors are used and adapted to the patient.22 PNE covers topics such as acute versus chronic pain, the purpose of acute pain, the origins of acute pain, the transition to chronic pain and potential factors that sustain chronic pain, explained from a biopsychosocial view rather than a pure patho-anatomical output, and as a consequence, patients’ illness perceptions also change to the biopsychosocial perspective.23 24 Previous single-arm studies using PNE in BCSs showed positive results on central sensitisation symptoms25 26 and pain-related function and quality of life,26 whereas results in a large randomised controlled trial comparing PNE combined with physical activity with biomedically focused education did not find any significant differences in different pain-related outcomes.27 However, the latter trial was conducted in BCSs who were not preselected for having significant pain, which might explain the absence of an added value of the respective PNE-based intervention.27 Moreover, all these previously conducted studies providing PNE to BCSs did not yet account for perceived injustice, which seems an important construct to target in people heaving feelings of injustice.
In addition to targeting perceived injustice during PNE, motivational interviewing can be used as a communication strategy throughout PNE to obtain behavioural change.28 Motivational interviewing is a directive, collaborative, patient-centred communication approach for eliciting and enhancing motivation for behavioural change by helping patients resolve ambivalence and uncertainty.29 30 Especially in people with perceived injustice, motivational interviewing techniques can be useful to shift the focus from that feeling of injustice to working on valuable life goals, changing their pain-related coping.31
Therefore, an innovative treatment, including PNE, focused on perceived injustice with the integration of motivational interviewing in BCSs with pain and perceived injustice. The primary objective of this study is to examine whether perceived injustice-targeted PNE is superior to biomedically focused pain education in reducing pain after 12 months in BCSs with perceived injustice and pain. The secondary objectives are to examine whether perceived injustice-targeted PNE results in improved quality of life and reduced perceived injustice and opioid use and can be found to be cost-effective as compared with biomedically focused pain education at 12 months of follow-up. The aforementioned objectives will lead to recommendations concerning the use of perceived injustice-targeted PNE combined with motivational interviewing in clinical practice. This is a protocol for a multicentre randomised controlled clinical trial with balanced treatment arms, 4 weeks of intervention and 0, 12 and 24 months of follow-up conducted in BCSs with perceived injustice and pain.
Methods and analysis
This protocol was written in accordance with the Standard Protocol Items: Recommendations for Interventional Trials recommendations32 and was registered prior to recruitment on ClinicalTrials.gov: NCT04730154. All items from the WHO Trial Registration Data Set can be found in online supplemental appendix 1 and all protocol versions in online supplemental appendix 2.
Supplemental material
Study design and study setting
This is a multicentred randomised controlled trial with two balanced treatment arms and with blinding of assessors and investigators. The trial is spread over five different hospitals in Flanders, Belgium (online supplemental appendix 3).
Eligibility criteria
To be eligible, participants must fulfil the definition of survivorship introduced by the European Organisation for Research and Treatment of Cancer (EORTC) Survivorship Task Force, in which a cancer survivor is as follows: ‘any person who has been diagnosed with cancer, has completed his or her primary treatment (with the exception of maintenance therapy) and has no evidence of active disease’.33 Additionally, participants need to:
be women aged ≥18 years;
be in complete remission and should have finished their primary breast cancer treatment with curative intent ≥3 months (adjuvant hormonal therapy and immunotherapy are tolerated);
report a pain severity of ≥3/10 on the Brief Pain Inventory (BPI);
be able to speak and read Dutch;
show a level of perceived injustice of ≥17/48 on the Injustice Experience Questionnaire (IEQ).
Participants will be excluded if they:
are diagnosed with new neoplasms or metastases;
have a chronic disease which is not well controlled and/or is causing pain (eg, fibromyalgia and rheumatoid arthritis);
are suffering from a severe psychological/psychiatric disease;
are suffering from dementia or cognitive impairment (unable to understand instructions and/or ≤11/28 on the Six-item Cognitive Impairment Test (6-item CIT));34 35
recently started a new treatment/training which is not yet at a stable level.
To facilitate participant recruitment and optimise the external validity of the study findings, assessments will take place at five different hospitals in Belgium (online supplemental appendix 3). Additionally, pharmacies, patient support groups, occupational health services, social media and advertisements in local newspapers will be used as recruitment strategies. The initial screening of interested participants will be executed by independent investigators at the hospitals or by phone. After this short screening, an online questionnaire (±5 min) will be sent to the participant to define the BPI pain severity score and the total IEQ score. Eligible patients will be asked to provide written informed consent (online supplemental appendix 4). With this consent, participants agree to keep appointments for treatment sessions, not to initiate any new treatment/medication from the moment they are contacted by phone for study inclusion until 3 months after the intervention or to participate in any other medical-scientific study during participation.
Interventions
To balance non-specific treatment effects, the duration, format, number of sessions and didactical approach will be identical in both treatment groups (see structured description of treatments in table 1). All participants will receive an online session (±1 hour) followed by three individual one-to-one sessions (±45 min), allowing them to individually tailor the sessions. After each session, all participants will be asked to report the content of the session in a personal logbook and to report co-interventions, medical consumption (eg, the type, dose, method of administration and frequency of medication) and any other healthcare visits and interventions. The one-to-one sessions are provided by trained physiotherapists at the study site of the participant’s preference and will be spread over a 4-week period.
Overview of the content of the sessions in both intervention groups
Treatment development and training
The treatment manuals, including the pre-recorded sessions, were developed before study initiation. The perceived injustice-targeted PNE and motivational interviewing manual were developed by combining research and the input of clinical experts in the field of PNE, perceived injustice and motivational interviewing. The biomedically focused pain education was developed by research experts in the field of breast cancer.
The training of the physiotherapist will be done by one of the developers of the manuals. Both trainings consist of a minimum of two contact moments of ±2 hours spread over ±3 weeks. The physiotherapists will be selected based on their experience and/or interest in working with cancer survivors. Moreover, experience with one of the two types of education provided in the trial was screened in advance in order to control for contamination (eg, a therapist with experience providing PNE cannot provide biomedically focused pain education).
Experimental treatment: perceived injustice-targeted PNE + motivational interviewing
The first online pre-recorded PNE session focuses on a better understanding of post-cancer pain prior to the first one-to-one session. The primary focus of the treatment plan is to shift maladaptive pain behaviour to beneficial pain behaviour. Therefore, the mechanisms underlying pain will be explained to clarify the rationale for changing. Perceived injustice will be introduced as a contributing factor for pain and central nervous system sensitisation. This can serve as a first step in accepting their condition and the associated suffering and should ease talking about perceived injustice during the next session. The content will be based on the books ‘Explain Pain’ and ‘Pijneducatie, een praktische handleiding voor paramedici’ as previously used in other chronic pain populations36 37 but adapted to BCSs.38 The information will be presented in different ways by using pictures, examples and metaphors.22 At the end of the first session, participants will have to read the perceived injustice-targeted PNE information leaflet summarising the information of the online session. Since an important portion of BCSs reports impairments in attention due to the so-called ‘chemo brain’,39 40 it will be valuable to have additional written information as well as the recorded session at the participant’s disposal to minimise the impact of loss of concentration.
Therapists will start the first one-to-one PNE session by discussing the participant’s responses to the online questionnaires, as well as their experience and questions regarding the online PNE session and the information leaflet. This information will be used to situate the participant within the phases of behavioural change, as well as to define perpetuating factors (eg, acceptance and opioid use) to start an individually tailored treatment. The practical guideline accounting for perceived injustice in cancer survivors will be used.31 Both valued life goals and treatment goals will be set within a shared decision-making process.
The second and third one-to-one PNE sessions will consist of individually tailored PNE based on the participant’s stage of change. The (perceived injustice-targeted PNE and) motivational interviewing will aim at encouraging participants in pursuing life goals again and restart valued occupations while experiencing pain by eliminating the feeling of wanting to control or avoid pain.15 41 In addition, pain acceptance will be addressed by broadening their understanding of their pain problem, including discussing the possible pain-aggravating role of anger and frustration. During the communication, following motivational interviewing principles, the therapist is supportive, empathetic, positive and hopeful and relies on the therapeutic alliance to assist in changing certain health behaviours based on the person’s internal thoughts such as perceived injustice, decisions and motivation. Motivational interviewing also aims to strengthen personal commitment by respecting the individual’s autonomy and assists in reaching a specific goal by exploring personal intentions or reasons for change.29 30
Active comparator: biomedically focused pain education
The first online pre-recorded biomedically focused pain education session will contain information about the different types of pain (nociceptive pain, neuropathic pain and nociplastic pain) and how oncological treatment methods, such as surgery, chemotherapy, radiotherapy and hormone therapy, are able to provoke pain with a primary focus on structural tissue damage. The role of different structures and injured versus healthy tissue in acute and persistent pain will be discussed. Pain will be explained from a biomedical perspective (eg, injured tissues causing pain) and a biomechanical point of view (eg, pain is explained as deviance from, eg, normal expected movement patterns and postures). At the end of the first session, participants will have to read the information leaflet from Stand Up to Cancer regarding ‘Pain in and after treatment’ summarising the information provided during the online session. The use of the leaflet within the study has been approved by Stand Up to Cancer.
During the first one-to-one session, participants’ responses to the online questionnaires and the participant’s experience with and questions regarding the online education module and the information leaflet will be discussed at the beginning of this session. After this, participants will receive accurate information about pain medication (eg, indication of use and adverse effects), if relevant, based on the cancer pain management proposed by WHO (World Health Organization).42
During the second one-to-one session, additional questions that arose after reading the information leaflet will be addressed. Second, additional pain treatment methods are explained, such as specialised techniques for nerve pain (eg, transcutaneous electrical nerve stimulation) and others (eg, physical activity). Therefore, participants will receive advice, including concluding treatment options, on how to deal with their pain. Goals will be set from a biomedical point of view.
The third one-to-one session will be used to support self-management. The latter will adhere to current best-evidence practice guidelines43–45 including advice on activity self-management (eg, to stretch muscles, increase their physical activity level gradually and tips regarding nutrition).
Outcome measures
The primary outcome is pain severity. The secondary outcomes are health-related quality of life, perceived injustice and outcomes for a cost-utility analysis. Sleep, fatigue, pain cognitions, depression, anger, acceptance, treatment adherence and compliance, and co-interventions will be added as explanatory outcomes. The outcomes are all self-reported questionnaires and based on the Dutch Oncoline recommendations and on previous studies performed in cancer46–54 and non-cancer pain populations.55–61 An overview of the assessments is presented in table 2. Assessments will be performed online at the following timepoints:
T0: within the week before the randomisation and the intervention (baseline)
T1: immediately after intervention
T2: 6 months after intervention
T3: 12 months after intervention (primary endpoint)
T4: 24 months after interventions (extended endpoint)
Study outcome measures by assessment time point
Personal characteristics
Personal characteristics including date of birth, nationality, race/ethnicity, level of education, professional situation, family income, relationship status, physical activity, smoking status, alcohol consumption, body mass index, comorbidities, lymphoedema, type of breast cancer treatments received, time since onset of complaints, time since complement of breast cancer treatment and treatment expectations will be collected at baseline.
Primary outcome: pain
BPI is developed by the Pain Research Group of the WHO Collaborating Centre for Symptom Evaluation in Cancer Care.62 It is a 14-item self-reported questionnaire assessing worst pain, pain severity and pain interference in patients over the past week on a scale of 0 to 10.62 Pain interference is measured as the average of the seven interference items. BPI is the most common, reliable and valid outcome measure to assess pain in cancer survivors (Cronbach’s alpha and test-retest reliability score >0.80).62
Secondary outcome measure: quality of life
Health-related quality of life is an established prognostic indicator of breast cancer.63 The EORTC Core Quality of Life Questionnaire (EORTC QLQ-C30) is a 30-item cancer-specific questionnaire developed for the assessment of the quality of life in patients with cancer.64 65 The EORTC QLQ-C30 is widely used in cancer studies, has been translated and validated in Dutch and shows acceptable psychometric properties.64 The internal consistency measured by Cronbach’s alpha resulted in 0.94.48
Secondary outcome measure: perceived injustice
The 12-item IEQ will be used to assess perceived injustice.66 Participants must rate the frequency of 12 different pain-related statements on a 5-point Likert scale ranging from 0 (not at all) to 4 (all the time). The sum of all items gives the total score which ranges from 0 to 48. The higher the score on the IEQ, the higher the level of perceived injustice. A cut-off value of 17 was calculated by taking the 75th percentile,66 which suggests a clinically relevant degree of perceived injustice in BCS. The IEQ has good (test-retest) reliability (ICC=0.86–0.87) in its Dutch version.67 The scores derived from the IEQ are found to be valid60 68 with an excellent internal consistency in advanced cancer.69
Secondary outcome measures for cost-effectiveness analysis
A cost-effectiveness analysis will be conducted following the manual by Hakkaart-Van Roijen et al.70
Healthcare use (including co-interventions) will be assessed using the Medical Consumption Questionnaire (MCQ).71 This is a well-established, generic, instrument-to-measure total (in-)direct medical consumption.71 Indirect costs will include costs related to productivity loss. These will be assessed using the Productivity Cost Questionnaire (PCQ).72 Both questionnaires are easy to use and able to generate valid data.73 74 In accordance with their user manuals, the questionnaires will be modified to match the respective setting.
Health-state utilities will be obtained from the EuroQol EQ-5D-5L and will be used to calculate quality-adjusted life years (QALY).75 76 The EQ-5D-5L items are scored on a 5-point Likert scale for five different dimensions.75 The EQ-5D-5L is a reliable and valid measurement tool for the evaluation of overall health in breast cancer.50 77 Moreover, Belgian population norms are available for the EQ-5D-5L.78 The use of the latter three tools is recommended by the Institute for Medical Technology Assessment, Erasmus University Rotterdam.79
Explanatory outcomes
A detailed overview of several explanatory outcomes which all have been proven to be related to the development of chronic pain80 can be found in table 3.
The explanatory outcomes with their measurement tool(s)
Treatment fidelity
Fidelity criteria will be developed before the start of the interventions. Independent investigators, experienced with the treatment, will evaluate a random selection of the tapes of each therapist using the fidelity criteria to score the audiotapes of the treatment sessions provided.81
Patient and public involvement
One of the biggest Belgian Cancer charities, Kom op tegen Kanker, reviewed all parts of the trial, including the design, management and conduct of the trial. We received input from their experience with patients who survived breast cancer. We carefully assessed the burden of the trial interventions on patients. We intend to disseminate the main results to trial participants and will seek patient and public involvement in the development of an appropriate method of dissemination.
Sample size calculation
Sample size calculations were performed with G*Power 3.1.3 for between-group differences (t test) at 12 months of follow-up. The sample size calculation was based on an earlier trial regarding a conservative intervention for treating pain in BCS that had the same primary outcome (eg, BPI) and identical 12-month follow-up.46 Based on that earlier trial46 and calculation methods described by Lakens,82 the effect size was set to 0.44, based on an observed difference of 1.8 on BPI and a CI between 0.9 and 2.6 for 83 participants. The type I error was set to 0.05 and the type II error to 0.2. The resulting sample size for a one-sided test was 65 per treatment arm. Accounting for a risk of loss to follow-up of 20%, a total sample size of 156 participants is needed.
Treatment allocation
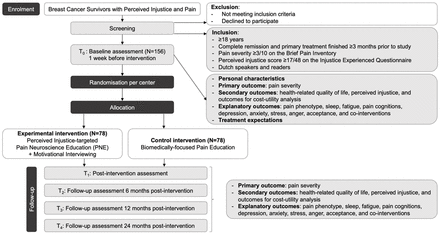
Randomisation (figure 1) will be done separately for each treatment centre by an independent researcher. Randomisation occurs using a computer-generated random number sequence (developed by Gerard E Dallal, PhD—http://www.randomization.com). A list with participant numbers and the group allocation that results from this randomisation procedure will be stored on a private SharePoint which is only accessible by the independent researcher. Participants will be scheduled by the therapists to receive their first assessment within 1 week of randomisation.
Flowchart of the BCS-PI trial (breast cancer survivor-perceived injustice trial).
Blinding
Due to the nature of the intervention, blinding participants to the content of the intervention is impossible. However, participants will not be informed about whether they received the experimental or control intervention. The statistician will be blinded to group allocation. All outcome measures are self-reported and eventual queries concerning the questionnaires will be addressed by a blinded independent assessor. The interventions will take place at different times during the day so that participants of different intervention groups do not meet and between-group contamination is avoided.
Data collection, data management and confidentiality
All questionnaires will be processed in an online software program REDCap.83 REDCap is General Data Protection Regulation compliant. Data, both numeric and textual, filled in by the participants online will be saved automatically. Encrypted identifiers (ie, a unique pseudonymised participant ID) will be used to separate the personally identifiable information from the clinical data. This link will be stored securely in REDcap. Clinical data will be saved under the pseudonymised participant ID. REDCap will be used for data storage, management and processing. Additionally, data will be stored on an encryption and password-secured Institutional SharePoint with sufficient storage space and limited access to the research team trained in human subject protection. Confidential individually identifiable data, including the pseudonymisation key, will be stored in a separate folder. Long-term data preservation will be done in the Vrije Universiteit Brussel University Archive.
To avoid loss to follow-up, REDCap automatically sends follow-up assessments and a reminder in case of no response for each endpoint. In addition, participants will be contacted by telephone and/or get reminders by email to complete all assessments.
Statistical analysis
A linear mixed model for repeated measures will be used to evaluate treatment effects over time in terms of pain, health-related quality of life, perceived injustice and opioid use. Such analysis allows more precise parameter estimates and can handle missing data. The baseline value of the outcome, explanatory outcomes (table 3), medication use and demographics will be considered covariates. Statistical and clinically significant differences will be defined, and the effect size will be determined. In addition, the numbers needed to treat will be calculated.
QALY’s (health effect) for the cost-effectiveness analysis will be calculated from utility scores derived from the EQ-5D-5L using Belgian population norms.78 Both direct healthcare costs (calculated based on the information gained from the MCQ) and indirect costs (productivity loss costs calculated based on the information gained from the PCQ)84 will be determined. Healthcare use will be valued using unit reference prices published by RIZIV-INAMI85 86 and BCFI-CBIP.87 All costs will be expressed in euros; indexed using the Health Index for Belgium, if necessary84; and reported in detail in a non-aggregated form. The incremental cost-effectiveness ratio will be calculated, and probabilistic sensitivity analysis will be performed. The impact of methodological choices will be evaluated by scenario analyses. Dissemination of the cost-utility analyses will follow the Consolidated Health Economic Evaluation Reporting Standards.88
Ethics and dissemination
Ethics
The agreement was obtained by all Ethics Committees, with the University Hospital Brussels as the Main Ethics Committee (B.U.N.1432020000068). Every modification will be sent to the Main Ethics Committee for approval. Participants included in the trial will be informed of any important modifications. All participants will provide informed written consent for their voluntary participation. They will always be able to withdraw from the study. No adverse effects are expected since this study includes no risk-involving measurements and treatments. Withdrawing from the study is possible at any time without the necessity to provide a reason for the withdrawal. If one intervention proves to be more effective than the other intervention (after total trial completion), participants will receive access to the most effective intervention. If participants do not properly follow the procedures, they may be withdrawn from the study prematurely.
Dissemination
The Consolidated Standards of Reporting Trials guideline89 will be used to report the findings. We will try to improve the knowledge of this topic area among researchers, patients (support groups), professional organisations and healthcare providers through presentations, conferences, social media campaigns, press and publications in journals. The funder will not be involved in or have any influence on the analysis and interpretation of the study results and will not impose any restrictions in terms of the dissemination of the study findings.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors JN was involved in all aspects of protocol design. PVW, RB, LL, DB, AT, MDC, AL, EH, ER and KM assisted with the design of the protocol respective to their expertise. EH and DB led the statistical aspects of the protocol. For the design of and training in the interventions, the following authors shared their expertise for specific components: JN, PVW, EH and LL, PNE; JN and PVW, motivational interviewing; RB, perceived injustice; and LL, biomedically focused pain education. ER was the lead author of the manuscript. All authors critically reviewed the manuscript and approved the final version for submission.
Funding This trial is funded by Stand Up to Cancer (Kom op tegen Kanker), a Belgian cancer charity (ANI251). Additional funding for operational costs is available from the Vrije Universiteit Brussel Chair of the Berekuyl Academy for Oncological Rehabilitation (JN). These funding sources had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data or decision to disseminate the results.
Competing interests JN and the Vrije Universiteit Brussel received lecturing/teaching fees from various professional associations and educational organisations. JN, PVW, LL and EH authored a book on PNE, but the royalties are collected by the Vrije Universiteit Brussel and not them personally.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.