Article Text
Abstract
Introduction Very preterm infants are at risk of abnormal microbiome colonisation in the first weeks to months of life. Several important associated factors have been identified including gestational age, mode of delivery, antibiotic exposure and feeding. Preterm infants are at risk of a number of pathologies for which the microbiome may play a central role, including necrotising enterocolitis and sepsis. The objective of this study is to determine detailed microbiome changes that occur around implementation of different management practices including empiric antibiotic use, advancement of feeds and administration of probiotics during admission to the neonatal intensive care unit.
Methods and analysis A single-site, longitudinal observational study of infants born less than 32 weeks gestation, including collection of maternal samples around delivery and breastmilk and infant samples from admission through discharge from the neonatal unit.
Ethics and dissemination The protocol was approved by the Clinical Research Ethics Committee of the Cork Teaching Hospitals.
The findings from this study will be disseminated in peer-reviewed journals, during scientific conferences, and directly to the study participants. Sequencing data will be deposited in public databases.
Trial registration number NCT05803577.
- Microbiology
- Neonatal intensive & critical care
- NEONATOLOGY
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
Study design will provide high resolution longitudinal microbiome data that will help to characterise the colonising microbiome of preterm infants, including the effect of, and progression after, treatments.
An array of samples will be collected including rectal swabs, stool, urine and blood from infants, peripartum maternal samples including stool, oral, skin, vaginal samples and expressed breastmilk from mothers.
This is a single-site study.
The study population is heterogenous, and the recruited sample size will be ~200 (mothers and infants).
Introduction
Over the last two decades, the materno-feto-neonatal microbiome has rapidly become an area of budding interest in the specialty of neonatology. While there is much debate as to whether a developing fetus is exposed to micro-organisms in utero,1 it is increasingly evident that the gut microbiome does not expand and diversify until the postnatal period at which point microbes from the mother and environment begin colonisation of the infant. Following birth, an initial relatively simple microbial community develops into a diverse microbial ecosystem in a controlled fashion over the first years of life.2 Bacterial colonisation, specifically in very low birth weight infants, may occur in an aberrant fashion for numerous reasons: an immature gut and immune system3 4; the environment in which they are nursed5; frequent exposure to antimicrobial agents6–8; and altered feeding practices such as delayed commencement of feeds or using formula in place of mother’s own milk.9–11 It is thought that the colonising microbiome of very preterm infants, and the factors that shape it, play a crucial role in the development of microbiome-mediated complications such as necrotising enterocolitis (NEC).
NEC is a complex multifactorial condition characterised by intestinal immaturity and dysbiosis in association with altered microvascular blood flow. It is the most commonly acquired serious gastrointestinal complication of prematurity and is rare in babies born above 32 weeks.12 Infants at risk are likely to have deficient intestinal mucus production, immature gut immunity and reduced endogenously produced antimicrobial factors. The ability of bacteria to adhere to epithelial cells is thereby enhanced, particularly after gut injury secondary to ischaemia/hypoperfusion. Although not fully understood, the most accepted theory for the pathogenesis of NEC is that in this setting, bacterial endotoxin binds to toll-like receptor 4 found on the intestinal epithelial cells and by so doing, pathogen-associated molecular pattern receptors are activated, resulting in cell apoptosis and epithelial injury.13 This leads to an intense inflammatory cascade in the exposed lamina propria, including activation of inflammatory cytokines such as tumour necrosis factor-alpha, interleukin (IL) 1 and IL-8, and ultimately necrosis of the surrounding tissue. Work by Stewart et al14 demonstrated that NEC tends not to occur in infants with a diverse gut microbiome. This may relate to inherent factors that make the individual less susceptible to NEC and simultaneously allow a diverse microbiome or may be due to protective effects associated with having a diverse gut microbiome, such as inflammatory modulation and improved gut epithelial integrity.
With early descriptions of the ‘ideal’ gut microbiome (ie, that of the vaginally delivered, exclusively breast fed term infant and without antibiotic treatment)15 16 and the identification of potentially deleterious bacteria harboured in the guts of the hospitalised very preterm infants15 17 18 came the advent of probiotics. Administration of these as a supplement to feeding aimed to lend some ‘normality’ to the gut microbiome of these vulnerable babies by supplanting potentially pathogenic bacteria and attempting to reduce the incidence of NEC.19–23 While probiotic preparations are widely available, shown to be safe24 25 and do, indeed, increase so-called beneficial bacteria present in the gut,26 the evidence for their benefit is contentious27 and practice varies from institution to institution.
Work has shown that the full term vaginally delivered and exclusively breastfed baby’s initial gut microbiome is dominated primarily by Actinobacteria28 29 while studies looking at preterm infants’ microbiome have shown a Proteobacteria (Bacilli dominance) and Firmicutes predominance.28 30–32 Clarification of the initial neonatal gut microbiome in the preterm population is confounded by the low bacterial biomass in early samples and high interindividual variability in composition. Individual medical management of each infant is likely to cause perturbations to the microbiome. Several factors associated with alterations in the neonatal gut microbiome have previously been identified. These include gestational age,28 mode of delivery,28 33 exposure to antibiotics (both prenatally and postnatally)6 and, importantly, feeding choice.34 Mothers of very low birth weight infants are typically encouraged to express breastmilk for their baby. This is associated with lower rates of sepsis and NEC.35 Some of these effects may be related to the bacteria or supporting molecules received in breastmilk from their mother.36 Correlations between changes in the neonatal gut microbiome and the microbiota of the milk they are being fed are limited and longitudinal analysis of breastmilk in this regard is lacking. Furthermore, interactions within breastmilk between bacteria and bacteriophage are not well characterised.
Given the rarity of some neonatal diseases, such as NEC, collaborative efforts are necessary to study data from a sufficient number of infants to draw robust conclusions.37 A number of prospective longitudinal repositories have been established, including the Great North Neonatal Biobank in the UK and the NEC Biorepository in the USA, with aims including the study and investigation of the preterm neonatal microbiome and it’s role in pathological disease processes such as NEC. Work stemming from these and others has considerably advanced our understanding of the microbiome in term and preterm infants over the past decade. Expanding data and sample collection to other regions will help in future collaborative work, to account for geographical variations in hospital practice and microbiome structure. This work will aim to longitudinally characterise the early and colonising intestinal microbiome of very preterm infants. We will analyse samples from infants less than 32 weeks gestational age at birth, with collection occurring longitudinally from birth to discharge from the neonatal intensive care unit (NICU). Microbiome composition will be evaluated using metagenomics and 16sRNA sequencing of stool. Bacterial function will be assessed longitudinally using untargeted metabolomics of infant urine. We will also interrogate some key perinatal maternal microbiome niches, including oral, vaginal, skin and intestinal, as well as expressed breastmilk to evaluate mother-infant transmission.
Methods
Study design
This single site study is a prospective longitudinal observational study of the intestinal microbiome of infants born between 23 and 32 weeks of gestation. It will investigate the establishment of the very premature infants’ microbiome using culture independent approaches, including the potential initial colonisation process of vertical transmission from mother to preterm infant, the apparent differences in establishment pattern based on feeding type, and the perturbations and recovery associated with different intensive care management strategies such as empiric antibiotic usage.
Study recruitment commenced in April 2020 and finished in December 2022. The primary endpoint of the study will be reached after the last enrolled participant completes 2-year neurodevelopmental follow-up.
Participant selection
Participants are infants, born between 23+0 weeks gestation and 31+6 weeks gestation, admitted to the Cork University Maternity Hospital (CUMH) NICU and their mothers.
The study was designed as a longitudinal observational study with a time-limited recruitment period of approximately 18 months. With a projected number of 100 infants less than 32 weeks to be admitted to the CUMH NICU annually, and an expected consent rate of 60%–70%, a pragmatically predefined minimum enrolment number of 80 participants over 18 months of recruitment was set.
Inclusion and exclusion criteria
To be eligible for the study, the participants must meet the terms of the inclusion and exclusion criteria as presented in table 1.
Inclusion and exclusion criteria of the Preterm Infants: Microbiome Establishment, Neuro-CrossTalk and Origins study
Recruitment
Pregnant women will be approached if there is a possibility of delivery before 32 weeks gestation. The study will be explained, and a participant information leaflet will be provided. Written informed consent will be obtained if the pregnant woman is agreeable after a sufficient amount of time to assimilate the information.
Multiple births will be enrolled separately. Samples will be collected from each infant but only one set of maternal samples would be collected. If one twin is delivered vaginally and the other by caesarean section, both skin and vaginal swabs will be collected from the mother if possible.
Written informed consent will be required for all participants. However, in exceptional circumstances (eg, precipitous delivery) participants may be enrolled with deferred consent with the intent that investigators would obtain written consent as soon as possible thereafter. This would facilitate in the event a mother was not available to provide written informed consent that samples could be collected, that is, a rectal swab from infant could be taken on admission to the NICU (as additional to the routine clinical swabs) and that, if passed a sample of the first meconium could be retained to ensure that, if subsequently enrolled, completeness of data for that participant could be maintained. Samples would be stored in a freezer on-site and would not be processed or analysed until written informed consent had been obtained. Similarly, out-born infants transferred to the study site may be enrolled with deferred consent and the infant’s first meconium and rectal swab retained until the mother is available to provide written informed consent. Again, sample processing or analysis would not be performed without written informed consent and the samples would remain frozen on site until that time.
Follow-up
Two-year neurodevelopmental assessment, using the Bayley Scale for Infant and Toddler Development, 3rd Edition (BSID-III), is routinely performed at CUMH for all infants born at 32 weeks gestational age or less.
Compensation
There is no compensation provided to the participants. There are no cost implications for the Health Service Executive or to the participants. The management of patients and investigative tests will comply with current standards of care.
Study timeline
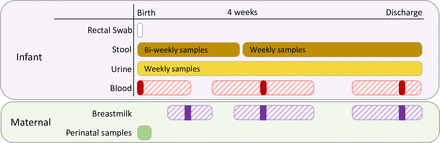
Each participant will have samples collected during the entirety of their neonatal unit stay (see the Patient and public involvement section and figure 1). Sample collection will not occur if the infant is transferred to another centre for ongoing management.
Sample collection timeline. Samples were collected from birth to discharge. Timing of discharge was determined clinically and was variable for each infant so no definitive timeframe was set. Solid bars represent timelines for regular sample collection. Dashed bars represent the ‘window of opportunity’ for sample collection for non-regular samples. Maternal perinatal samples, collected within 4 days of delivery, included stool, oral, skin and vaginal swabs.
Participant withdrawal/exclusion
Under the Declaration of Helsinki, the researcher will explain to the participants that they have the right to withdraw from the study at any time and that this will in no way prejudice their future treatment. The reason for withdrawal will be recorded in the source documents and on the appropriate CRF. Withdrawn participants will be replaced. With consent samples from individuals withdrawing from the study will be stored and may be analysed in the study. Participants excluded from the study after consenting will be replaced.
Ethics and dissemination
The study is conducted following the version Fortaleza, Brazil, October 2013 of the Declaration of Helsinki 1964. The Protocol and the Informed Consent Form have been approved by the Clinical Research Ethics Committee of the Cork Teaching Hospitals (CREC) before commencement (approval letter ECM 4 (t) 10 November 2020 and ECM 3 (mmm) 9 March 2021). On 25 August 2022 Protocol version 5 has been approved by the CREC (approval letter ECM 4 (t) 10/11/2020 and ECM 3 (vvv) 20 September 2022).
If a protocol amendment is necessary, this will be prepared with the agreement of the chief investigator and signed by the relevant parties. If the amendment is substantial, it will be submitted to the CREC and possibly other public bodies according to local requirements for review and approval. The protocol amendment will not be implemented before the required approvals are obtained.
The trial is sponsored by University College Cork (College Road, Cork T12 K8AF, +353 (0)21 490 3000). The sponsor is not involved in study design; collection, management, analysis and interpretation of data; writing of the report; and the decision to submit for publication.
The findings from this study will be disseminated in peer-reviewed journals, during scientific conferences and directly to the study participants.
Patient and public involvement
None.
Objectives and outcomes
Primary objective
Detailed characterisation of the very preterm microbiome from birth to final hospital discharge, using culture independent approaches, including maternal origin and initial colonisation, postnatal alterations secondary to medical management (eg, antibiotic and probiotic administration) and recovery/progression after discontinuation.
To describe changes in community structure and function of gut microbiome in preterm infants during stay in the neonatal unit.
To compare microbiome successional colonisation in infants requiring varying degrees of initial respiratory support.
To evaluate probiotic strains in the gut microbiome using next generation sequencing during probiotic commencement, colonisation and persistence after discontinuation.
Secondary objectives
To determine changes in the human milk microbiome using culture independent approaches over time with increasing postmenstrual age and characterise alterations in microbial content and investigate the influence of breastmilk-derived microbes in the infant gut microbiome.
Exploratory objectives
To assess metabolic functioning and systemic indicators in relation to microbiome composition at the time of sampling.
To analyse maternal-infant transmission by correlation of the maternal microbiome with the early colonising microbiome of enrolled infants.
Sample collection
An overview of the sample collection timeline is presented in figure 1.
Stool samples and rectal swabs
Rectal swabs are taken routinely on admission to the NICU for clinical microbiological screening. These are carried out by the admitting clinical nurse. One extra rectal swab will be taken for microbiome analysis at this time. Stool sampling will be carried out at the cotside during the course of the neonatal stay, a sample of the first meconium passed will be collected from the nappy and thereafter stool will be sampled from the infant’s nappy bi-weekly during routine cares. After 4 weeks of age, samples will be collected fortnightly. Samples are placed in a freezer in the neonatal unit immediately after sample collection. Thereafter samples are transferred to a −20°C freezer on-site for cataloguing prior to transfer to the off-site laboratory (APC Microbiome, Ireland, University College Cork, Ireland) for further processing and storage.
Urine samples
During the inpatient stay, urine will be collected weekly, if possible, the attending neonatal nurse by placing sterile cotton balls in the infant’s nappy which will then be removed at the next occurrence of routine cares. Urine is squeezed from the cotton balls into sterile collection bottles. Urine samples are placed in neonatal unit freezer immediately after collection, subsequently catalogued and transferred to the on-site −20°C freezer, and finally transferred to the off-site laboratory.
Blood samples
Infant blood samples will be collected from infants at times when clinical blood sampling is being performed. These will be collected in Serum Gel Z micro sample tubes. A sample volume of 1% of the estimated blood volume (estimated blood volume=80 mL/kg) will be collected. The sample will be immediately centrifuged on-site for 8 min at 9000g and then frozen at −20°C in the on-site freezer. Thereafter, the blood sample will be transferred to the off-site laboratory for further storage and processing. If possible, two to three blood samples will be collected during the entire neonatal stay.
Breastmilk samples
In the case where mothers are expressing milk for their infants, breastmilk samples will be collected once there is enough supply to provide for the infant’s intake and surplus for storage. Breastmilk is expressed by mother’s either on-site in the neonatal unit or at home into sterile collection bottles. Expressed breastmilk is refrigerated immediately and can be stored refrigerated for up to 24 hours or frozen. Five to fifteen millilitres of breastmilk is collected from the refrigerated breastmilk storage bottles using aseptic technique and transferred to sterile cryogenic storage tubes. Samples are catalogued and placed in an on-site −20°C freezer immediately after collection, and subsequently transferred to the off-site laboratory thereafter. If possible, three breastmilk samples will be collected during the entire neonatal stay.
Maternal stool samples
Within 3 days of delivery, mothers will be approached to provide a sample from their first stool passed after delivery. Once collected, the sample will be brought by the mother to the neonatal unit when it will be placed in the neonatal unit freezer. Samples will be collected from there to be catalogued and transferred to an on-site −20°C freezer, and thereafter, to the off-site laboratory.
Maternal oral samples
Oral swabs will be collected from mothers within 3 days after birth. Samples will not be collected within 1 hour after mother’s have eaten food. Samples are catalogued and placed in an on-site −20°C freezer immediately after collection, and subsequently transferred to the off-site laboratory thereafter.
Maternal skin swabs
In the case of infants delivered by caesarean section, skin swabs will be collected from mothers within 3 days after birth. Swabs are wetted with sterile water for injection prior to swabbing the skin of the left wrist. Samples are catalogued and placed in an on-site −20°C freezer immediately after collection, and subsequently transferred to the off-site laboratory thereafter.
Maternal vaginal swabs
In the case of infants born by vaginal delivery mothers will be asked within 3 days of delivery to provide a vaginal swab. Samples are catalogued and placed in an on-site −20°C freezer immediately after collection, and subsequently transferred to the off-site laboratory thereafter.
Sample analysis
Faecal samples will be processed for DNA extraction and metabolomics. DNA will be extracted from stool using the modification of the Repeated Bead Beating Plus Column (RBB+C) method38 and subjected to 16S rRNA and/or shotgun sequencing. Metabolomics of infant’s faeces will be analysed by the gas chromatography–mass spectrometry technique using methyl chloroformate derivatisation.39 Bioinformatic analysis will identify the microbial composition at the phylum, genus and family levels using both read-based40 and assembly-based41 approaches. Metagenomic data will be correlated with physiological and clinical parameters and biomarkers. Advanced statistical and bioinformatics approaches will be applied to identify modifiable environmental factors that influence bacterial groups/consortia. This includes diversity analysis,42 differential abundance analysis,43 strain persistence and vertical transmission44 and genome-scale metabolic modelling.45
Breast milk samples will be used for DNA extraction (using modification of Qiagen DNeasy Powerfood Microbial Kit (QIAGEN, Manchester, UK) and the milk microbiome composition will be analysed by using state-of-the-art methods, possibly including but not limited to sequencing, and quantitative PCR.
Urine metabolomics analysis will be analysed by the liquid chromatography–mass spectrometry technique.28
The maternal oral microbiome composition will be analysed by using state-of-the-art methods after DNA extraction, possibly including but not limited to sequencing, and quantitative PCR. The bioinformatic analysis will identify the microbial composition at the phylum, genus and family levels.
From vaginal swabs, DNA will be extracted using the modification of the RBB+C method38 and the vaginal microbiome composition will be analysed by using state-of-the-art methods, possibly including but not limited to sequencing, and quantitative PCR. The bioinformatic analysis will identify the microbial composition at the phylum, genus, and family levels.
Adverse events and participant well-being
This is an observational study. No investigational medicinal product will be administered to study participants at any stage during the study. The study procedures are not greater than minimal risk, adverse events (AEs) and serious AEs are not expected. All self-reported AEs (AEs) will be listed documenting duration, severity, participant outcome, and if any therapy was required. The study chief investigator will review these and advance any reports to the local ethics committee if it is deemed necessary.
Data collection and management
Data collection/Case Report Forms
Data will be collected throughout the neonatal stay and from clinic visits after discharge. Data will be extracted from the electronic health records of each subject meeting the eligibility criteria and being included in the study. Data will be recorded in an electronic spreadsheet data collection tool stored on an encrypted password protected computer and backed up to the secure university server. All study staff responsible for entering data into the data collection tool will be trained prior to the start-up of the study.
The data in the Case Report Forms (CRFs) will be consistent with the relevant source documents. The only source documents that will be available will be participants’ in-patient electronic health record at CUMH. All data stored will be pseudonymised and treated with strict confidentiality in accordance with the General Data Protection Regulation.
Sequencing data
Data types and formats collected and/or produced will include Raw whole genome sequencing (WGS) data (fastq), WGS assembly files and excel file with strains name (fasta), WGS annotation files (fna, gff, faa, ffn), 16S raw data (fastq), 16S ASV table (csv), 16S ‘phyloseq’ file, which includes metadata for each sample (rds), Raw shotgun data (fastq), MAGs/bins from shotgun data (fna), Pangenomes files (newick, fa, csv/tab) and pipeline for the metabolic modelling part and generated data (sbml, mat). Sequencing data will be deposited in public databases.
Statistical analysis
Sample size justification
This study was designed as a longitudinal observational trial with the primary aim of detailed characterisation of the colonising microbiome of very preterm infants and, additionally, determination of factors relating to perturbations seen therein. As such, there was no statistically predefined sample size but instead the goal to recruit the largest sample population possible in the allotted timeframe for study recruitment, in this case 18–20 months. Post-hoc, we will calculate and state statistical power analysis to give a representation of statistically relevant sample size for this cohort.
Discussion
This study aims to longitudinally observe the gut microbiome of very preterm infants during their admission to CUMH neonatal unit. We intend to characterise in detail how clinical management practices influence the microbiome of these infants, including but not limited to delivery practices, antibiotic usage, feeding types and regimens, and probiotic usage. Very preterm infants have increased risk of potentially microbiome-mediated pathologies such as NEC and late-onset sepsis. The role that the microbiome plays in these diseases may relate to the overall composition of the microbiome, individual species within the microbiome, or temporal changes that occur. As such, management practices may be influential in the development of microbiome structure and may therefore have an indirect role in the development of these diseases.
At CUMH, probiotics (Bifidobacterim bifidum and Lactobacillus acidophilus) are administered to all infants born less than 32 weeks gestation or less than 1500 g birth weight from when feeding is deemed to be tolerated until 34 weeks corrected gestational age. We intend to observe these probiotic strains within the gut microbiome community, how they persist after supplementation is discontinued, and how clinical management may affect their colonisation.
Collected maternal samples will allow us to study transmission of microbes to premature infants and longitudinally collected breastmilk samples will allow us to study how the milk microbiome plays a role in shaping gut microbiome structure of the preterm infant.
The authors acknowledge the limitations of this study design. It is a single centre observational study and the results may not be generalisable across all other institutions. As all infants that are to be enrolled in the study will receive probiotic supplementation, there will not be a control group without probiotics with which to compare. The study is designed as descriptive and without intervention. Accordingly, the management of the infants enrolled has not been standardised. While the clinical demographics of the enrolled infants will be described in careful detail, all confounders may not be accounted for owing to variability in clinical courses and the variability in management decisions made by the various providers caring for the infants.
We envisage that this work will add to the already existing information on the preterm microbiome. As neonatal care practices can differ markedly from institution to institution we hope that this study will provide novel insights into how the microbiome is established, including maternal transmission of microbes at delivery and through breastmilk. This study will inform our understanding of microbial colonisation of very preterm infants, successional patterns of colonisation and how clinical management practices influence these. It will also provide valuable information on probiotic colonisation and practices around probiotic administration and may help future development of efficacious probiotic strains.
Status of study
The study is ongoing as of 7 April 2021. Prospective recruitment of new participants was completed on 31 December 2022. The primary endpoint will be when 2-year neurodevelopment follow-up has been completed for the final participant. At the time of publication, no sequencing data from samples collected has been received or analysed.
Ethics statements
Patient consent for publication
Acknowledgments
The authors would like to thank all the study participants and the neonatal nursing staff in Cork University Maternity Hospital.
References
Footnotes
Contributors DH wrote the manuscript. DH, PR, CS and EMD were involved in study conception and design, acquisition of ethical approval, and review of the manuscript. DH coordinated participant recruitment, sample collection, on-site sample processing and sample transport. SW coordinated laboratory sample processing in conjunction with AKW. GG coordinated bioinformatic analysis. All authors read and approved the final manuscript.
Funding This publication has emanated from research conducted with financial support of Science Foundation Ireland (SFI) under Grant No. 12/RC/2273_P2 and 19/SP/6989.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.