Article Text
Abstract
Introduction Knowledge of the clinical liver anatomy has evolved with advanced imaging modalities and laparoscopic surgery. Therefore, precise anatomical resection knowledge has become the standard treatment for primary and secondary liver cancer. Segmentectomy, a parenchymal-preserving approach, is regarded as an option for anatomical resections in patients with impaired liver. Indocyanine green (ICG) staining is a promising method for understanding the anatomical borders of the liver segments. There are two methods of ICG staining (positive and negative), and the superiority of either approach has not been determined to date.
Methods and analysis This is a prospective randomised controlled superiority clinical trial performed in a single centre tertiary hospital in Japan. A comparison between the accuracy of positive and negative ICG staining in guiding laparoscopic anatomical liver resection is planned in this study. Possible candidates are patients with liver malignant tumours in whom laparoscopic monosegmentectomy or subsegmentectomy is planned. Fifty patients will be prospectively allocated into the following two groups: group A, ICG-negative staining group, and group B, ICG-positive staining group. The optimal dose of ICG for positive staining will be determined during the preparation phase. To assess the ability of the ICG fluorescence guidance in anatomical resection, the primary endpoint is the success rate of ICG staining, which consists of a subjective optical scoring (SOS) based on three components: superficial demarcation in the liver surface, visualisation of the parenchymal borders and consistency with the preoperative three-dimensional simulation. The secondary endpoints are the evaluation of short-term surgical outcomes and recurrence-free survival.
Ethics and dissemination The study was approved by Ageo Central General Hospital Clinical Research Ethical Committee (No: 1044) and it carried out following the Declaration of Helsinki (2013 revision). Informed consent will be taken from the patients before participating. The findings will be disseminated through peer-reviewed publications, scientific meetings and conferences.
Trial registration number UMIN000049815.
- Positive staining
- Negative staining
- Indocyanine green
- Laparoscopic anatomical liver resection
- Glissonean pedicle
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Positive staining
- Negative staining
- Indocyanine green
- Laparoscopic anatomical liver resection
- Glissonean pedicle
STRENGTHS AND LIMITATIONS OF THIS STUDY
Performing a pilot study prior to the clinical trial helps to more accurately determine the appropriate dose of indocyanine green.
As a limitation, the staining technique is operator-dependent; therefore, a definitive conclusion cannot be drawn solely from this single-centre trial.
The blinded randomised nature of the trial will reduce bias resulting from subjective assessments by the operators.
The study is conducted at a single centre; therefore, this might limit the generalisability of the results.
Our study population will consist of patients with malignant liver tumours; hence, the results might not be applicable to other pathological conditions.
Introduction
Since the previous three international consensus meetings (Louisville, Iwate and Southampton),1–3 laparoscopic liver resection (LLR) as a treatment for the chronic liver disease has developed considerably worldwide. Understanding clinical liver anatomy has gained increasing attention with the advancement of three-dimensional (3D) simulation software. The emergence of indocyanine green (ICG) fluorescence and high-quality magnified view of laparoscopic imaging has also contributed to the knowledge of clinical liver anatomy to a large extent. Therefore, more precise liver resections, such as anatomical liver resections (ALR), have recently been more commonly performed based on liver inflow and outflow. Currently, ALR is accepted as a standard therapy for liver cancer because of its oncological effectiveness. However, even if we do not consider the long-term efficiency of ALR, the watershed of the segments/sections is easy to transect because of the sparse vessels in the intersegmental/sectional planes. Besides, leaving fewer ischaemic areas in the remnant liver is considered reasonable after ALR.
Makuuchi et al reported a new concept of small ALR (ie, segmentectomy in Brisbane terminology 2000), which applies well to the therapeutic principle in the Asia Pacific region, where most hepatic malignancies are hepatocellular carcinoma arising in the impaired liver.4 Thus, a small ALR was established based on the parenchyma-sparing principle in maldistributed patient groups. Since the 1990s, when laparoscopic surgery has developed noticeably, anatomical knowledge of the internal and external liver has gradually increased owing to its magnified and unique caudal/dorsal view. Clinical questions regarding the landmarks for the segmental borders and approach for the tumour-bearing portal pedicles were discussed during the 32nd meeting of the Japanese Society of Hepato-Biliary-Pancreatic Surgery held in Japan in 2021, and the new terminology for the small ALR was described by updating the Brisbane 2000 terminology5–7 (table 1).
The Tokyo 2020 terminology of liver anatomy and resections: updates of the Brisbane 2000: system
ICG fluorescence imaging is considered helpful for the real-time identification of segmental boundaries during liver parenchymal transection in LLR, achieving the concept of anatomical parenchyma-sparing resection.8 To assess the real-time haemodynamics, ICG fluorescence has not yet been matched in the field of intraoperative imaging modality owing to its unique excretion(through bile juice) characteristic and deep penetration of approximately 1 cm. However, the optimal usage (ie, the dose and timing for multiple uses) has not yet been clarified according to its various uses in each report. Two methods of ICG staining have been reported based on its administration routes: positive and negative staining.9 Although Wakabayashi et al addressed the optimal dose and timing of ICG application for positive and negative staining,10 the superiority of either staining method has not been determined to date (figure 1).
Summary of practical doses and timing of injection. *Passed on experience, florescence technology and patient conditions. CRLM, colorectal liver metastasis; HCC, hepatocellular carcinoma; ICGR15, indocyanine green retention test after 15 min.
It is of utmost importance to accurately dissect the anatomical boundaries between the tumour-bearing liver segment and adjacent segments in the case of ALR with ICG fluorescence guidance. Funamizu et al reported a positive correlation between the estimated and actual liver volumes (ALRV) after the ICG negative staining approach.11 ICG-negative staining can precisely delineate the anatomical borders during resection, maintaining both radical resection and sufficient healthy parenchyma. Additionally, Chiow et al reported preferable clarity of ICG fluorescence guidance in the two approaches of staining in robotic ALR.12 However, the results depended only on subjective assessment, and the outcomes were never statistically compared between the two staining approaches.
This study aims to compare the accuracy of liver segmentation using positive and negative staining during LLR to achieve precise ALR, such as segmentectomy, based on preoperative planning. Furthermore, future research can be conducted on the long-term outcomes of precise ALR.
Methods and analysis
Study design
This prospective study is a randomised controlled superiority clinical trial on patients with malignant liver lesions who will undergo segmentectomy using ICG fluorescence imaging navigation. This study will be conducted at the Ageo Central General Hospital (Saitama, Japan), a referral centre for LLR in Japan.
Pilot trial
A small-scale pilot study will be performed on six patients (12% of the sample size of the main study) to determine the appropriate dose of ICG-positive staining.
Hypothesis
We hypothesise that there is a statistical difference between the success rate of staining and short-term outcomes of the positive and negative ICG staining approaches in performing precise LLR. Theoretically, ICG-negative staining is a more solid approach for liver segmentation than ICG-positive staining. To perform ICG-negative staining, the Glissonean approach advocated by Takasaki is reasonable because the inflow of tumour-bearing areas is completely blocked before liver transection.13 This concept is based on a non-touch isolation technique for malignant tumours. However, to our knowledge, no available literature has specifically examined the potential benefit of negative staining compared with positive staining in laparoscopic segmentectomy.
Target population
Patients with primary or metastatic liver tumours planned for monosegmentectom and subsegmentectomy from February 2023 to December 2025 will be candidates for this clinical trial. The following inclusion and exclusion criteria are created to unify the selection of patients in this study. The inclusion criteria are as follows: male or female patients with solitary primary or metastatic liver tumours, aged ≥18 years, scheduled for elective LLR, preserved liver function, ability to understand the nature of the study, and willingness to join and provide voluntary written consent. The liver functional reserve will be evaluated by serum biochemical tests (albumin level, total bilirubin level and prothrombin time) and ICG retention rate at 15 min (ICG-15R). The severity of the liver disease will be assessed based on Child-Pugh stages and liver damage classification defined by the Liver Cancer Study Group of Japan.14 Preserved liver function is defined as an ICG-15R less than 30% and a Child-Pugh classification A or B. The exclusion criteria are as follows: repeat liver resection, multiple tumours, concomitant resection of other organs, severe liver or renal insufficiency, ICG hypersensitivity, pregnancy or breast feeding and inability to understand the nature of the study or refuse it. The schematic representation of the algorithm for this project, which has been designed with close consideration of the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines,15 16 is shown in online supplemental file 1 .
Supplemental material
Sample size calculation
The sample size was calculated using the EpiCalc 2000 software (Gilman and Myatt, 1998).17

where α=0.05, (1-β) = 0.95.
Although no previous data are available in the literature to compare the negative and positive ICG staining, we decided to use the data reported by Chiow et al.12 Thus, P1 (percentage of cases that had clear demarcation with positive ICG)=50% (6 out of 12) and P2 (percentage of cases that had clear demarcation with negative ICG)=92.5% (37 out of 40) are set in the power calculation. Consequently, the minimum required sample size is 25 in each group, with a total of 50 patients. Investigators may enrol more participants to avoid a significant decrease in the study power caused by attrition bias.
Randomisation and blinding
A randomised controlled superiority trial will be performed at the Ageo Central General Hospital. Fifty patients will be randomly assigned (1:1) to receive either positive or negative ICG staining. The minimisation method will be used for the randomisation dividing the participants into two groups. In addition, ICG-15R will be used as a parameter to equalise the background liver function to minimise the intergroup bias. Tumour aetiology will be also equalised between the groups. Allocation concealment will be performed until the patients are enrolled and assigned to the operation.
Intervention and surgical procedures
The preoperative routine test and planning for the patient have been described elsewhere.18 ICG-R15 tests will be conducted 2 weeks before surgery to assess patients’ hepatic reserve using an ICG dose of 0.5 mg/kg. A 3D vascular simulation models are constructed by a specific workstation (ZIOSTATION 2, Ziosoft, Tokyo, Japan), depending on the multidetector slice CT. Surgical planning is fashioned in line with the ‘cone unit’ theory instead of Couinaud’s stratification. Furthermore, preoperative volumetry will measure the total liver volume (TLV) and estimated liver resection volume (ELRV). To examine the accuracy of LLR, the ALRV will be calculated by dividing the actual liver resection mass (g) by standardised liver density (1.05 g/mL).11 19 Finally, the discrepancy between the ELRV and ALRV will be calculated as |ELRV-ALRV|/TLV×100 (%). We will use the 1688 Advanced Imaging Modalities Platform (Stryker, Michigan, USA) as the laparoscopic near-infrared camera throughout the designated study period. The extrahepatic (extrafascial) Glissonean approach will be used in all patients involved in this study to encircle the target Glissonean pedicle supplying the tumour following the preoperative simulation. Liver parenchyma division will be performed using a Cavitron Ultrasonic Surgical Aspirator (CUSA, ValleyLab, Colorado, USA). During the extrahepatic Glissonean approach, the 3D simulation model will be repeatedly referred to on a screen to ensure that the targeted pedicle tree is addressed.
Operative procedure for group A
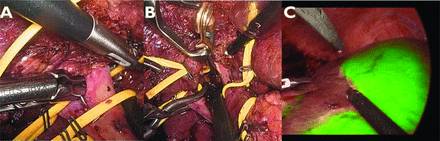
During the early phase of surgery, the extrahepatic (extrafascial), Glissonean approach will be performed to encircle the target Glissonean pedicle, feeding the tumourous area, corresponding precisely to the preoperative simulation (figure 2). To avoid postoperative bile leakage, it is essential to transect towards the liver parenchyma instead of the Glissonean sheath using the CUSA. During the extrahepatic Glissonean approach, the 3D simulation model will be repeatedly referred to on a primary screen to correct the pedicle tree. When identified, the target pedicle will be clamped using an endoscopic bulldog to make the diseased area completely ischaemic. Sequentially, inflow blockage will be confirmed using laparoscopic intraoperative ultrasonography with Doppler mode. Since the staining is irreversible after ICG injection, 0.15 mL/kg ultrasound contrast medium (SONAZOID, Daiichi-Sankyo, Tokyo, Japan) will be systematically injected before ICG injection. If the target area is adequately cyanosed, 0.5 mg/body ICG will be intravenously injected in the ICG-negative staining method. The demarcation line appears as a border between the colour-coded and non-colour-coded areas, marked on the liver surface. In the deeper parenchyma, the intersegmental plane can also be coded by the ICG fluorescence emission, which corresponds to the course of transection. The 1688 Advanced Imaging Modalities Platform will be used for the near-infrared camera system in all cases. This system has an overlay mode that enables the user to superimpose an ICG fluorescence image to a white-light image. This mode facilitates precise parenchymal transection according to the border between the colour-coded and non-colour-coded areas. Liver transection will be performed using the CUSA and other energy devices.
Indocyanine green (ICG) negative staining for colorectal liver metastasis in segment 5 and 6. (A) Dissection between the Laennec’s capsule and Glissonean sheath and identification of right posterior and anterior Glissonean pedicles. (B) Dissection continuing ahead liver parenchyma and clamping the Glissonean pedicle 5 and 6 with applying bulldog forceps. (C) After the administration of indocyanine green into peripheral vein, the demarcation line is identified.
Operative procedure for group B
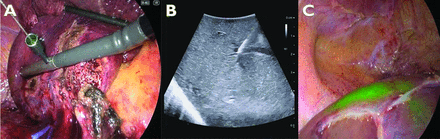
On the contrary, in ICG positive-staining, ICG will be directly injected into the portal branches responsible for resected territories or surrounding territories to visualise the clear demarcation planes (figure 3). The portal branches of the tumour-bearing liver segments will be targeted and punctured under ultrasound guidance with an 18 or 21 gauge spinal or percutaneous transhepatic cholangio-drainage needle introduced through the abdominal wall. The needle hole will assist the direction of the needle in a dedicated laparoscopic ultrasound probe (provided by BK Medical, Herlev, Denmark). Subsequently, a small volume of ICG (1 mL of 0.025 mg/mL) will be slowly injected into the portal branch to avoid the risk of ICG retrograde flow into the neighbouring segments with undesired staining without clamping the hepatic artery. Liver transection will be performed using CUSA and other energy devices.
Indocyanine green positive staining for colorectal liver metastasis in segment 7. (A) Identification and puncture of portal venous branch 7 (P7) under the guidance of intraoperative ultrasound. (B) Ultrasound findings after puncturing the P7. (C) Identification of the demarcation line between segment 7 and the adjacent segments after ICG injection into the P7.
Primary endpoint
To determine the ability of the ICG fluorescence guidance in anatomical resection, the primary endpoint will be the success rate of ICG staining, which consists of a subjective optical scoring (SOS) based on three components: superficial demarcation in the liver surface, visualisation of the parenchymal borders and consistency with the preoperative 3D simulation. Each criterion is scored on a scale of 0–2 (max 6 points). We will compare the scores between two groups (group A and group B) using a t-test to determine if there are significant differences in the effectiveness of the intervention. It is also subjective to estimate the resection margin and shape of the specimen in comparison with the preoperative and postoperative 3D simulations of the liver.
Secondary endpoints
The secondary endpoints will be the short-term surgical outcomes, such as the operative time, blood loss and complication rates. Recurrence-free survival at 1 year will also be addressed. The patients will be followed up at the outpatient clinic after surgery every 3 months with regular laboratory and radiological assessments using CT and MRI.
Data collection
The data will be collected in four phases: pilot, preoperative, operative and postoperative (table 2). In each phase, specific information will be collected for assessment. All the phases will be digitally recorded and reviewed by the authors.
Schedule of participation, investigation and assessment, preoperative findings and 12-month follow-up
Phase 0 (pilot study)
To determine the best dose of ICG to be administered for positive staining, a preliminary study of six patients will be performed as the first step. The initial trial dose will be 0.025 mg/mL, and the first patient will be administered 1 mL of this dose. Each successive patient will receive one extra millilitre of ICG at the same dose until sufficient positive staining is achieved. Since positive staining can potentially lead to overstaining due to ICG reperfusion, we have imposed a maximum limit of 3 mL for the ICG injection to minimise the impact of overstaining.
Phase 1 (preoperative period)
The databases will be extracted from patient charts, which include the following baseline characteristics: age, sex, body mass index, American Society of Anesthesiologist physical status, underlying liver comorbidities, chronic hepatitis status, preoperative chemotherapy, tumour size and location, volumetric and biochemical laboratory investigations including liver function test, ICG-15R and tumour markers.
Phase 2 (operative period)
This stage contains anaesthetic, technical (surgical) and pathological parameters. The anaesthetic parameters include the ICG dose, route and anaphylactic reactions, if any. The ICG dose will be determined in accordance with the findings from phase 0. The surgical parameters include the SOS components, blood loss, operative time and intraoperative complications. The pathological parameters include the histopathological diagnosis, largest tumour size, margin status (R0≥1 mm) and weight.20 21
Phase 3 (postoperative period)
The postoperative period will focus on early and late complications. It will be graded according to the extended Clavien-Dindo classification of surgical complications, published by the Japan Clinical Oncology Group, which describes the original criteria of the Clavien-Dindo classification more specifically.22The first follow-up visits will be conducted 2 weeks after hospital discharge and every 3 months after that. Follow-up assessment will be performed by adapting routine blood tests, including liver function tests, coagulation function tests, tumour markers and abdominal CT and MRI.
Study timeline
Data will be collected between February 2023 and December 2025, and statistical analysis will be completed after December 2026. Participants will be officially informed about the study during their preoperative visit to our clinic; therefore, they will have an extended period to choose to participate. Possible complications will be evaluated 12 months after the surgery. The outline of enrolment, interventions and follow-up assessments are described in table 2.
Data monitoring
The data will be monitored by frequently checking whether the study is being carried out safely by the proposed algorithm and whether the information is precisely collected. The following items will be reviewed every 3 months: informed consent (obtained and signed), participant retention, study implementation system, security, data and the progression in the process.
Statistical analysis of outcome measures
Data will be analysed using IBM SPSS Statistics for Windows V.29 (IBM). The general characteristics of the participants will be summarised using descriptive statistics. The χ2 test will be used to analyse categorical data. T-test will be used to analyse continuous data. Analysis of variance and logistic regression tests will be used to test the hypothesis and compare the groups using the baseline values as covariates; the choice of the test will depend on the type of response variables. To compare recurrence-free survival, Kaplan-Meier curves will be plotted, and a log-rank test will be performed. Statistical significance will be set at p<0.05. Interim succinct will not be included in this project.
Safety analysis
The safety endpoint of this study is the incidence of adverse events. A chart will be prepared to determine the endpoints. A two-sided 95% CI will be calculated to estimate the proportion of adverse events.
Patient and public involvement
There is no intention to select or specify any patient or citizen to participate in the planning of this study.
Ethics and dissemination
Is there any scientific and clinical value in conducting this study?
The ICG fluorescence imaging system plays a significant role in laparoscopic liver surgery because of the illustration of transection surfaces during parenchymal resection. We aim to evaluate the efficacy and safety of performing subsegmentectomy/monosegmentectomy, using the two techniques of the ICG-staining imaging system, by assessing the association between the success rate of identifying hepatic segments and clinical outcomes. This study will help determine the staining technique that can achieve precise resection and fewer complications. Theoretically, this is expected to reflect the improvement in outcomes and patient safety positively. This study is the first to compare the accuracy of these two staining procedures. We believe that the results will point towards the method for performing precise laparoscopic liver segmentectomy and subsegmentectomy. This study is expected to establish a milestone for the indications of each staining method to achieve the best outcomes and broaden our scientific experience in laparoscopic liver surgery. To enhance objectivity influenced by staining techniques, future trials may be necessary to determine the appropriate dosage for each staining, taking into consideration the variations among near-infrared camera settings.
Ethical approval
The study has been approved by the Ageo Central General Hospital Clinical Research Ethical Committee (approval number: 1044) and will be carried out in accordance with the Declaration of Helsinki (2013 revision).23 If any adjustment must be made during the study process, information will be sent to the Ageo Central General Hospital Clinical Research Ethical Committee. Informed consent is to be obtained from all participating patients. This ensures that all participants involved in the study will receive comprehensive information about the study’s objectives, procedures, potential risks and benefits before their participation. Ethical considerations and strict adherence to informed consent protocols are of paramount importance to safeguard the rights and well-being of the participants throughout the research process. The requirements for participation include age 18 years or older, preserved liver function and willingness to be included in the study (An example of the participant consent form can be found in online supplemental material 2).
Supplemental material
Participants’ rights, safety and disadvantages
All authors and contributors involved in the study are committed to maintaining each patient’s privacy. No identifying factors will be divulged in the study. Very little information that is only relevant to the case will be included, but without risking the exposure of patients’ identities. We will assign an identification code for each subject in the study to ease access to all data and documents.
Foreseeable disadvantages (burdens and risks)
To date, ICG administration is not known to cause many serious side effects.24 However, anaphylactic reactions may occur in a few patients. Our patients will be followed up for adverse events and pre-examined for any health conditions that might precipitate or aggravate any resulting complications. We will inform the patients before the procedure about the possible side effects and management plans once they develop. They will also be informed about the need to postpone or cancel the procedure and surgery if any contraindications or complications arise.
Ethics statements
Patient consent for publication
Acknowledgments
We would like to acknowledge the effort of Dr. Mawaddah Ababnih regarding the revision of the article by reading and proofreading multiple times for language errors and arrangement of sentences.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors MAMA has contributed to the conception and design of the work, acquisition of data, drafting the work and revising it critically for important intellectual content, final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. TW has contributed to the conception and design of the work, analysis and interpretation of data, revising the work critically for important intellectual function, final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MC has contributed to the conception and design of the work, analysis and interpretation of data, drafting the work and revising it critically for important intellectual content, final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. KM has contributed to the conception and design of the work, acquisition of data, drafting the work and revising it critically for important intellectual content, final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. YF has contributed to the conception and design of the work, acquisition of data, drafting the work and revising it critically for important intellectual content, final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. EA has contributed to the conception and design of the work, significantly contributed to the analysis and interpretation of data for the work, drafting the work and revising it critically for important intellectual content, final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. GW has contributed to the conception and design of the work, acquisition of data, drafting the work and revising it critically for important intellectual content, final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding Japanese Foundation for Research and Promotion of Endoscopy, Award/Grant no: NA.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.