Article Text
Abstract
Introduction Three-dimensional (3D) total body photography may improve early detection of melanoma and facilitate surveillance, leading to better prognosis and lower healthcare costs. The Australian Centre of Excellence in Melanoma Imaging and Diagnosis (ACEMID) cohort study will assess long-term outcomes from delivery of a precision strategy of monitoring skin lesions using skin surface imaging technology embedded into health services across Australia.
Methods and analysis A prospective cohort study will enrol 15 000 participants aged 18 years and above, across 15 Australian sites. Participants will attend study visits according to their melanoma risk category: very high risk, high risk or low/average risk, every 6, 12 and 24 months, respectively, over 3 years. Participants will undergo 3D total body photography and dermoscopy imaging at study visits. A baseline questionnaire will be administered to collect sociodemographic, phenotypic, quality of life and sun behaviour data. A follow-up questionnaire will be administered every 12 months to obtain changes in sun behaviour and quality of life. A saliva sample will be collected at the baseline visit from a subsample.
Ethics and dissemination The ACEMID cohort study was approved by the Metro South Health Human Research Ethics Committee (approval number: HREC/2019/QMS/57206) and the University of Queensland Human Research Ethics Committee (approval number: 2019003077). The findings will be reported through peer-reviewed and lay publications and presentations at conferences.
Trial registration number ACTRN12619001706167.
- DERMATOLOGY
- Adult dermatology
- Epidemiology
- Dermatopathology
- PUBLIC HEALTH
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The Australian Centre of Excellence in Melanoma Imaging and Diagnosis cohort study will establish the largest, world-first, prospectively collected standardised three-dimensional total body skin surface image data collection.
Comprehensive collection of data on personal, immunological, genetic and clinical risk factors.
Large sample size, recruited across 15 metropolitan and regional sites in three Australian eastern states.
Central and western Australia are not represented, which could lead to under-representation of Indigenous Australians.
Introduction
Australia has the highest incidence rate of melanoma in the world, in both males (42 per 100 000 person-years) and females (31 per 100 000 person-years).1 Melanoma is the most common invasive cancer in Australians aged 20–39 years and in the whole population it is the third most commonly diagnosed cancer in both males and females.2 The lifetime risk of being diagnosed with invasive melanoma tripled in Australia between 1982 and 2020, mainly driven by increasing incidence among the older population, and despite melanoma incidence decreasing in younger generations who have benefited from sun protection campaigns.
The burden of melanoma on healthcare systems is significant and increasing.3 The number of melanoma-related hospitalisations increased by 63% during 2002–2014,4 and melanoma and keratinocyte skin cancers (including basal cell carcinoma and squamous cell carcinoma) represent over a quarter of all cancer-related hospitalisations in Australia. In addition, people living in regional or remote Australia are often disadvantaged in their access to primary healthcare and diagnostic services, which may adversely affect prognosis.5 6 In 2021 in Australia, the mean first-year costs of melanoma per patient ranged from $A644 (95% uncertainty interval (UI) $A642 to $A647) for melanoma in situ to $A100 725 (95% UI $A84 288 to $A119 070) for unresectable stage III/IV disease. Australia-wide direct health system costs for newly diagnosed patients with melanoma were just under $A400 million.7 Furthermore, the quality of life for people diagnosed with melanoma and their family and friends has been shown to be negatively impacted, especially due to the fear of melanoma recurrence.8 Identifying melanomas at an early stage is associated with good prognosis,9 leading to reduced healthcare costs and better quality of life. Difficulties in accurately diagnosing melanoma and other skin cancers also contribute to many additional excisions for benign lesions, leading to additional costs for people and the healthcare systems.10 11
The clinical diagnosis of skin cancers relies heavily on visual observation of the skin surface with the assistance of dermoscopy. Clinical images can be used to monitor skin lesions, document locations of skin lesions and monitor response to treatment. Dermatologists have traditionally employed direct visual assessment, clinical memory recall and, if available, two-dimensional (2D) digital images of the skin lesions. However, this approach is imperfect and manipulation of a three-dimensional (3D) surface such as the human skin into a 2D format can compromise accuracy. Composing a body map of a patient via 2D imaging is also time-consuming; it requires multiple separate images of the patient to be taken in a variety of different anatomical positions that may overlap or conversely fail to include lesions that have not been captured from a specific camera angle.
Our team has established the Australian Centre of Excellence in Melanoma Imaging and Diagnosis (ACEMID). The 3D total body imaging technology that is the screening modality for the ACEMID cohort study will allow for objective and secure imaging and collection of 3D avatars, which will be cleansed and stored securely on a national research repository.12 Such 3D avatars enable documentation of the skin surface (except soles of feet, scalp and areas covered by underwear) to unprecedented levels of detail. Here, we describe the protocol for the ACEMID cohort study that uses 3D total body photography to monitor lesions for the early detection of melanoma over a 3-year period.
Methods and analysis
Study design and setting
This is a prospective population-based cohort study, stratifying participants based on their calculated risk of melanoma, to undergo risk-appropriate regularity of 3D total body photography of skin lesions over a 3-year period. The delivery of 15 total body 3D imaging systems with integrated data infrastructure will be implemented across metropolitan and regional areas in the states of Queensland (QLD), New South Wales (NSW) and Victoria (VIC) in Australia.
The main aims of this study are to:
Establish a large prospectively collected standardised 3D total body skin surface image database with tagged dermoscopy, together with scanning of pathology slides collected from excised lesions and other relevant participant information, including genomic data and Medicare claims data.
Develop novel diagnostic algorithms using the image repository to overcome the current high degree of variability in diagnostic accuracy of melanoma and other skin cancers.
Prospectively validate available melanoma risk tools to inform individualised risk-stratified screening and surveillance programmes for the Australian population.
Reduce the overarching burden, morbidity and mortality associated with melanoma, by helping ensure that specialist skin cancer services are targeted effectively and equitably to Australians most in need.
Assess patient acceptability of new technologies, including 3D total body skin surface imaging, telehealth and novel diagnostics, and examine the impacts of these modalities on quality of life.
Model the potential quality of life benefits and cost savings to the patient and the healthcare system associated with a targeted and accurate screening approach.
Study population
This study will recruit 15 000 people aged 18 years and over, across 15 sites across three eastern States of Australia at varying latitudes and in metropolitan and regional locations.
Patient and public involvement
Prior to obtaining funding for this project, members of the public previously involved in skin cancer research were invited to provide feedback to help inform our research plan. The ACEMID Project formed a Consumer Working Group consisting of key researchers, representatives from melanoma advocacy groups and consumer representatives. This group has been heavily involved in the study design and progress and will continue to contribute for the duration of the study. A consumer representative will also be a member of the ACEMID Executive Committee and consumers will take part in each of the three State Steering Committees. We will also conduct regular consumer forums to keep participants and the public involved in our research. Furthermore, participants will also receive regular updates on the progress of the study and other information via a study newsletter and a study website, to aid study retention.
Inclusion criteria
Australians are eligible to participate in the ACEMID cohort study if they are aged 18 years or older, willing to attend multiple study visits and have a medical practitioner that can be contacted by the study team. People at any level of melanoma risk are eligible to participate.
Recruitment
A risk-stratified sample of participants will be recruited using several channels, including medical referrals, social media and traditional media (such as television news reports). Participants from previous and existing skin cancer research studies will also be invited to participate in this study. People who would like to participate are able to register their interest by sending an email to the study email address or via the contact form on the study website.
People who express interest to participate in the study or are referred to participate in the study will be contacted via telephone. At clinical sites, potential participants may be recruited during their dermatology appointments. During this contact (via telephone or in clinic), individuals will be provided with study information. If the potential participant would like to proceed, their eligibility and their risk for melanoma will be assessed, and participants will be enrolled until each risk category is filled.
Melanoma risk group and intervention
At enrolment, participants will be stratified into three groups: low/average, high and very high risk. All individuals with a history of melanoma will be allocated to the very high-risk group as their risk of developing a subsequent primary melanoma is very high compared with the general population.13 For people without a history of melanoma, we will categorise risk levels using a validated melanoma risk tool14 that is available online through the Melanoma Institute of Australia (https://www.melanomarisk.org.au/FirstMelLand). The online risk tool provides an individual’s personal risk (lower than average, average, higher than average) that approximately corresponds to the lower quarter, middle 50%, and top quarter, respectively, of the Australian population melanoma risk distribution, based on traditional risk factors (hair colour, naevi, family history of melanoma, personal history of keratinocyte skin cancer, sunbed use), and in comparison to someone of the same age and gender and living in the same State.
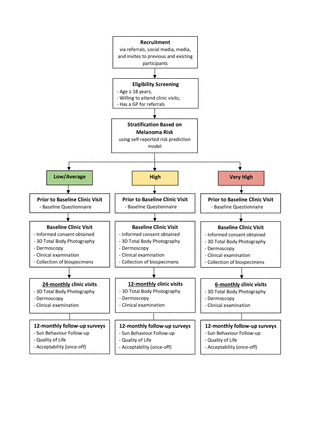
Participants with a low or average risk of melanoma will undergo 3D total body photography and dermoscopy at baseline and at 24 months (two study visits). Participants identified as having high risk of melanoma will be seen at the clinic for repeat 3D total body photography and dermoscopy every 12 months (baseline and three annual study visits). Participants in the very high-risk group will undergo 3D total body photography and dermoscopy every 6 months (baseline and six subsequent study visits) (figure 1). The ACEMID medical practitioner will have discretion to see participants return sooner than scheduled to monitor any changes in suspicious skin lesions.
Overview of study procedures. (3D: three-dimensional; GP: general practitioner).
Baseline study procedures and data collected
At the baseline study visit, research staff will discuss the participant information and consent form with potential participants and give them an opportunity to ask any questions. If consent is obtained, participants will undergo 3D total body photography, dermoscopy, a skin examination for research purposes only and may be asked to provide a saliva sample.
Questionnaire
Participants will be asked to complete a baseline questionnaire either prior to attending the baseline study visit or during the study visit. This baseline questionnaire will collect information on personal and demographic data, history of sun exposure, sun protection strategies, skin check and skin cancer history, perceived melanoma risk and quality of life. Health-related quality of life will be collected using the Australian Assessment of Quality of Life four domains (AQoL-4D) instrument.15–17 Participants who have a history of skin cancer will also complete the Skin Cancer Quality of Life Impact Tool (SCQOLIT).18
Clinical data
Melanographers and/or dermatologically trained medical practitioners will conduct a research skin examination. Eye colour and freckling density on the face, dorsum of right hand and shoulders will be recorded (none, mild, moderate, severe). Skin colour will be clinically assessed as being very fair, fair, medium, olive, brown or dark brown on the ventral upper arm (unexposed) and dorsal forearm (exposed), using a standard colour chart. Skin colour will also be assessed objectively using the 3D total body photography once validated algorithms for this purpose have been developed. Height and weight of participants will be measured. Information relating to any suspicious lesions will be recorded, including recent changes in size, shape, elevation, bleeding, itch, previous biopsy, previous cryotherapy or other ablative treatments.
3D total body photography and dermoscopy
Participants will undergo 3D total body photography (excluding skin beneath underwear, scalp and soles of feet) using a VECTRA whole-body scanner (VECTRA WB360 Serial Number WB00009, Canfield Scientific, Parsippany, New Jersey, USA). The scanner consists of 92 cameras with white or cross-polarised lighting, which simultaneously capture images to construct a digital 3D avatar of the participant, providing a record of all pigmented and non-pigmented skin lesions.
Each participant will undress to their underwear (underwear that exposes the most skin that the participant is comfortable with) with hair tied up if applicable. Participants will be instructed on the correct stance and posture for scanning; the 3D image will then be captured in approximately 5 s and the 3D avatar digitally will be constructed in approximately 10 min. Participants may be offered their images on a password-protected Universal Serial Bus device, for their reference and to take routine visits to their medical practitioner if required.
Dermoscopic images (non-polarised or polarised depending on site dermatologist’s discretion) will be taken of any skin lesions that are of concern to the study participant or dermatologically trained melanographer using a Visiomed D200e dermatoscope (Canfield Scientific) attached to the VECTRA. Any lesions 5 mm or greater in diameter will also be dermoscopically imaged, with a maximum of 50 lesions per participant.
Collection of biological samples
A convenience sample of participants will be asked to provide a saliva sample. Saliva samples will be collected using an Oragene DNA self-collection kit (DNA Genotek) according to the standards recommended by the Royal College of Pathologists of Australasia for medical genetic testing (Position Statement 3/2007), adopting the guidelines for Specimen Labelling at Point of Collection (1/2006). Depending on the available study resources, some participants may also be asked to provide a blood, tissue or urine sample at the baseline visit, as approved by the ethics committee. Donation of biospecimens including saliva, blood, urine and/or tissue samples is voluntary, and will not affect the participant’s inclusion in the cohort study if they do not wish to donate biospecimens.
Collection of healthcare use
Medicare claims data through linkage from Services Australia has been approved (Services Australia EREC approval RMS1199) for 4.5 years of data from the participant’s baseline visit date. This will include Medicare item number and description, provider charge, schedule fee ($A), patient out-of-pocket cost and prescribed medicines, for the duration of the study period. These deidentified data will be used to analyse the type, volume and cost of healthcare used per participant and for the cohort. Consent for these data is optional and will be obtained via a separate consent form.
Follow-up study procedures
Participants will undergo repeat 3D total body imaging, dermoscopy and clinical examination every 24, 12 or 6 months based on if they are in the low/average risk, high risk or very high risk group, respectively (figure 1). At the 12-monthly follow-up visits, a follow-up questionnaire will be administered to assess changes in sun behaviour, skin cancer diagnoses and quality of life. Participants in the low/average group will complete their 12-month and 36-month questionnaire online. For all risk groups, at the 24-month follow-up visit, a questionnaire on the acceptability of 3D total body photography will also be administered to obtain feedback on this technology.
Identification of suspicious lesions
During the study period, lesions identified by the melanographer or study dermatologist as suspicious for malignancy will be referred to the participant’s clinician for management. A report including images of any lesions requiring management will be sent to the participant’s nominated treating medical practitioner. Any referrals will be followed up and pathology reports requested.
Participants are encouraged to continue seeing their clinician for routine skin checks. At follow-up study visits, participants will be asked if they have had any skin lesions excised by their regular clinician outside of the study. Pathology reports of these excised skin lesions will be requested, and this information will be recorded.
As per routine clinical practice, skin biopsies and excisions will be sent to accredited pathology laboratories to be processed and diagnosed using standard histology practices. Pathology reports of all skin biopsies or excisions in study participants will be collected by the study team. Following the generation of a pathology report, some of the slides used for microscopic diagnosis may undergo digital scanning (all melanomas, borderline lesions and 10% of benign lesions will be randomly selected). Whole slide scans will be reviewed by 2–3 pathologists to confirm diagnoses.
Participant reimbursement
Monetary reimbursements will not be provided to participants in this study.
Standard Operating Procedure adherence checks
Adherence at sites to the Standard Operating Procedures of the study will be monitored on a quarterly basis to ensure standardised quality of data produced at each of the 15 sites. These checks will be conducted by the respective ACEMID managers in QLD, NSW and VIC.
Data collection and storage
Clinical and questionnaire data will be recorded on the Research Electronic Data Capture (REDCap) system, a secure study database software built by Vanderbilt University and hosted by Monash University, Melbourne, Australia (https://redcap.helix.monash.edu).
Pathology reports will be obtained from each State’s (Queensland, New South Wales, Victoria) cancer registry and pathology laboratories; redacted and uploaded onto the REDCap database. Claims and health service data will be collected by linkage from the Medicare Benefits Schedule (MBS) and the Pharmaceutical Benefits Scheme (PBS), via a separate consent form approved by Services Australia.
Cleansed image data (deidentified and/or without demographic data) will be stored in the national ACEMID research repository. Access to the research repository will be controlled and governed by the ACEMID Data Governance Working Group using secure software platforms, including Extensible Neuroimaging Archive Toolkit (XNAT) and relevant Universities’ Secure eResearch Platforms (SeRP) and Keypoint.
Participant data will be handled with utmost confidentiality. A participant log will be kept where a coded ID number will be allocated to each participant, which will be kept securely and separately from other data. All images, questionnaires, forms, medical reports, samples and genetic data will only have the participant ID number to protect their privacy. Participants may withdraw from the study at any time and will be given the option for the study to either retain or destroy any collected data.
Data processing and analysis
Sample size
The ACEMID cohort study aims to recruit 15 000 participants, with quota sampling employed to recruit 3000 participants (20%) in the low/average melanoma risk group, 9000 participants (60%) in the high risk group and 3000 participants (20%) in the very high-risk group.
Based on previous studies, from baseline to 36 months, we anticipate a participant dropout rate of up to 20%, 10% and 10% in the low/average, high and very high-risk groups, respectively.
Based on the melanoma incidence in previous studies conducted in NSW and QLD,19 20 we conservatively expect to observe a melanoma incidence rate over the 3-year study follow-up of 0.4% (n=8), 2% (n=162) and 5% (n=135) in the low/average, high and very high-risk groups, respectively.21
Power calculations were conducted to ensure the sample size is sufficient to detect associations between new melanomas (yes/no) and potential risk factors in both the high-risk and very high-risk groups. Based on the numbers above, we will have at least 80% power to detect a relative risk of 2 comparing risk factor presence and absence, for a risk factor that has prevalence 10% in each of the high and very high risk groups, assuming a two-sided alpha of 0.05. In analyses for which multiple comparisons may be of concern, for example, broad-based genetic markers, a more stringent alpha may be appropriate. In this case with a lower alpha (0.001, two sided), we will have at least 80% power to detect a relative risk of 2.3 in the high-risk group, and 2.5 in the very high-risk group, for a risk factor with prevalence of 10% in each group.
We expect a small number of new melanomas in the low/average melanoma risk group, and therefore, we examine power for associations between risk factors and new keratinocyte cancers in this group. Based on an expectation of approximately 67 keratinocyte cancers per 1 melanoma,22 we anticipate 6.7% of the low/average risk group will develop a new keratinocyte cancer over 2 years of follow-up. Based on this, we will have 89% power to detect a relative risk of 2, for a risk factor that is prevalent in only 10% of the low/average risk group with 89% power and assuming a two-sided alpha of 0.05. As above, for analyses in which multiple comparisons may be of concern, with a two-sided alpha of 0.001, there is 91% power to detect a relative risk of 2.5, for a risk factor that is prevalent in only 10% of the low/average risk group.
Statistical analysis
Primary outcome
For the cross-sectional data, descriptive statistical analyses including counts and proportions will be used to describe the total number of in situ and advanced melanoma, keratinocyte cancers and pigmented and non-pigmented skin lesions (≥2 mm and ≥5 mm). These skin lesions will be analysed and summarised according to melanoma risk group, sex, age and body site. Descriptive statistics will be used to summarise dermoscopic features of pigmented skin lesions by body site. Cross-sectional associations will be quantified by regression methods suited to the measurement scale of each outcome.
Secondary outcomes
To assess the unadjusted and adjusted strength of association between participants’ phenotypic or genotypic characteristics and lesion outcomes, linear and log-linear (relative risk) regression models will be fitted, for continuous and binary/count outcomes, respectively. Where multiple lesion outcomes per participant are included the model for mean outcome will be fit using generalised estimating equations with an exchangeable working correlation matrix and robust standard errors. The adjusted models will include explanatory factors (eg, skin type) according to a causal diagram. Interaction terms will also be fitted where appropriate (eg, phenotype and genotype) to explain lesion counts or distributions.
Additional longitudinal analysis will include spatiotemporal models to analyse lesion distribution patterns over time, and whether changes are associated with a naevus cluster, or a specific body site, in each of the risk groups. Age, sex and residence-based geographical and socioeconomic status related information are available to assess response bias effects. To further assess effects of response bias, we will compare traits including naevus counts to those of other studies in well-characterised community and clinical populations.
Performance of melanoma and keratinocyte risk prediction models based on self-reported risk factors will be assessed throughout the study period, overall and in different population subgroups. Discrimination will be assessed using area under the receiver operating characteristic curve at a single time point and c-index with censored data, and net reclassification improvement. Calibration will be assessed using calibration plots. Objective measures of phenotypic risk from imaging, together with behavioural and genomic risk factors, will be assessed in their ability to further discriminate risk of developing melanoma and keratinocyte cancers.
Exploratory outcomes
Macroscopic, dermoscopic and histological imaging data, lesion history and deidentified participant risk data will be stored in a national research repository for development of diagnostic algorithms. Machine learning, including convolutional neural networks and support vector machine algorithms will be applied to training data sets and evaluated in test sets. The impact of including metadata, including genomic data and other risk factors, on diagnostic accuracy, will be assessed in these analyses. The relative benefit of including macroscopic and dermoscopic data with histology image data will also be assessed.
Genomic analysis
The saliva samples will be processed to extract DNA. Once extracted, the DNA will be quantified using either the Nanodrop spectrophotometer or the Qubit 2.0 fluorometer along with the Qubit dsDNA BR Assay kits (Thermo Scientific). The DNA will be stored at a temperature of −20°C. A minimum of 2 µg of the extracted DNA will be submitted for genotyping.
Economic analysis
Utility-based quality of life will be assessed using scores from the AQoL-4D instrument at baseline, 12 and 24 months for the cohort during the study period, and used to calculate quality-adjusted survival at 24 months. Data relating to treatment costs, hospitalisations, participant time and effort required to participate, convenience of location, acceptability of the technology, out-of-pocket expenses and changes in productivity associated with referrals will be collected. Total costs, including use of artificial intelligence (AI)-based diagnosis, and total outcomes expressed as quality-adjusted life-years (QALY) gained for this cohort, will be calculated and reported as mean values with SD and 95% CIs. A comparison of healthcare use 12 months prior and 48 months after enrolment in the cohort study will be undertaken using MBS and PBS data. Mean annual and total healthcare use, costs and QALYs will be quantified for the cohort, preinception and postinception. A budget impact analysis (BIA) will be modelled using best practice methods23–25 over a 5-year time frame. In this BIA, several scenarios will be investigated for the provision of services in a staged implementation in which 50% of eligible Australians will access 3D total body photography in year 1, subsequently rising to 60% in year 2, 70% in year 3, 80% in year 4 and 90% in year 5.
Ethics and dissemination
Ethics approval has been obtained by the Metro South Human Research Ethics Committee (approval number: HREC/2019/QMS/57206) and the University of Queensland Human Research Ethics Committee (Approval number: 2019003077). The trial is prospectively registered on the Australian New Zealand Clinical Trials Registry (ACTRN12619001706167). The protocol adheres to the Standard Protocol Items Recommendations for Interventional Trials statement.26 The findings from this study will be circulated through peer-reviewed publications, conferences, policy documents and media outlets.
Ethics statements
Patient consent for publication
Acknowledgments
The authors would like to thank the staff at every participating ACEMID site for their valuable contributions to this research.
References
Footnotes
Contributors UK, AEC, PF-P, CH, GM, VM, RM, HPS, RW, MS, GK, EP, RAM and MJ were all involved in developing the study protocol. AEC, PF-P, GM, VM, RM, HPS, RW, MJ and others worked together on the funding proposal. RW provided support for the development of the statistical analysis plan. RM and RAM provided the health economics analysis. All authors have reviewed, edited and approved the final version.
Funding This research was conducted with the support of the following: Australian Cancer Research Foundation (ACRF); NHMRC Clinical Trials and Cohort Studies Grant (2001517); NHMRC Centres of Research Excellence (2 006 551 and APP1135285); and NHMRC Synergy Grant (2009923). AEC received an NHMRC Investigator Grant (2008454). RLM is funded by a University of Sydney, Robinson Fellowship and an NHMRC Investigator grant (APP1194703). MJ is funded by a NHMRC TRIP Fellowship (APP1151021). HPS holds an NHMRC MRFF Next Generation Clinical Researchers Programme Practitioner Fellowship (APP1137127).
Disclaimer The funders had no role in the study design, data collection, data analysis, manuscript preparation and/or publication decisions.
Competing interests HPS is a shareholder of MoleMap NZ and E-derm-Consult and undertakes regular teledermatological reporting for both companies. HPS is a Medical Consultant for Canfield Scientific, Blaze Bioscience and a Medical Advisor for First Derm. VM has received speakers fees from Novartis, Bristol Myers Squibb, Merck, Janssen and conference sponsorship from L’Oreal. PF-P has consulted for Leo, Amgen, Abbvie, UCB, Sanofi, Janssen, Novartis, BMS, MSD, Pfizer and Lilly. PF-P has received speakers fees from Janssen, Leo, Amgen, Sanofi, Lilly, Abbvie, UCB, Novartis, Merck and Pfizer.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.