Article Text
Abstract
Objective We aimed to construct and validate a prognostic nomogram to predict cancer-specific survival (CSS) after surgery in patients with advanced endometrial carcinoma (EC).
Design Retrospective cohort study.
Setting and participants The Surveillance, Epidemiology, and End Results (SEER) Database contains cancer incidence and survival data from population-based cancer registries in the USA. A total of 5445 patients from the SEER Database diagnosed with advanced EC between 2004 and 2015 were included and randomised 7:3 into a training cohort (n=3812) and a validation cohort (n=1633).
Outcome measure CSS.
Results The nomograms for CSS included 10 variables (positive regional nodes, age, tumour size, International Federation of Gynecology and Obstetrics (FIGO) stage, grade, ethnicity, income, radiation, chemotherapy and historical stage) based on the forward stepwise regression results. They revealed discrimination and calibration using the concordance index (C-index) and area under the time-dependent receiver operating characteristic curve, with a C-index value of 0.7324 (95% CI=0.7181 to 0.7468) and 0.7511 (95% CI=0.7301 to 0.7722) for the training and validation cohorts, respectively. Using calibration plots, a high degree of conformance was shown between the predicted and observed results. Additionally, a comparison of the nomogram and FIGO staging based on changes in the C-index, net reclassification index and integrated discrimination improvement demonstrated that the nomogram had better accuracy and efficacy.
Conclusions We successfully constructed an accurate and effective nomogram to predict CSS in patients with advanced EC, which may help clinicians determine optimal individualised treatment strategies for patients with advanced EC. The predictive performance of the nomogram was evaluated thoroughly, but only internally. Therefore, further validation using different data sources is warranted in future related studies.
- Gynaecological oncology
- Surgical pathology
- Radiation oncology
- Gynaecological oncology
- Health economics
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request. Based on the SEER website (https://www cancer.gov/policies/accessibility), the National Cancer Institute (NCI) provides access to all individuals seeking information on http://www.cancer.gov. The NCI SEER Database is publicly available. Data supporting the findings of this study are available from the corresponding author upon request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The Surveillance, Epidemiology, and End Results (SEER) Database is a large database with sufficiently large numbers of samples.
The SEER Database lacks laboratory test data, which may influence the prognoses of patients with advanced endometrial carcinoma.
The chemotherapy and radiotherapy information contained in the SEER Database can only be obtained by signing legal agreements that are currently unavailable.
This study may have suffered from selection bias, as all cases were retrieved from the same database.
Our nomogram’s predictive performance was evaluated thoroughly, but only internally; external validation using different data sources is warranted.
Introduction
Endometrial carcinoma (EC) is the sixth most common cancer in women, with 417 000 new cases diagnosed worldwide in 2020.1 There are two histological types of EC.2 3 Type I tumours include those with grade 1 or 2 endometrioid histological classifications, accounting for approximately 80% of ECs. Type II tumours account for 10–20% of ECs, and include grade 3 endometrioid tumours and tumours with non-endometrioid histology. EC is primarily treated surgically, with radiation and chemotherapy as common adjuvant modalities. For patients with EC who undergo surgery, adjuvant therapy determines disease recurrence for risk stratification based on tumour stage, tumour histology and other pathological factors. There is overwhelming evidence that traditional pathological features such as histopathological type, grade, myometrial invasion and lymphovascular space invasion (LVSI) are imperative for assessing prognosis.4 Molecular classification in high-grade and/or high-risk ECs shows that POLE-mutated tumours have an excellent prognosis, p53-abnormal tumours have a poor prognosis, and ECs with mismatch repair (MMR) deficiency or non-specific molecular profile have an intermediate prognosis.5 The latest European (European Society of Gynaecological Oncology/European SocieTy for Radiotherapy and Oncology/European Society of Pathology 2020)/American (National Comprehensive Cancer Network 2020) guidelines combining traditional pathology and The Cancer Genome Atlas (TCGA) molecular groups have proposed a novel risk stratification model: low, intermediate, high–intermediate, high and advanced metastasis.6 Generally, the 5-year survival rates are 80–90% and 70–80% for stage I and II ECs, respectively, and 20–60% for stage III and IV ECs.7 8 Stage III and IV ECs are classified as advanced or high-risk ECs. Patients with advanced and recurrent EC have poor prognoses, with an expected 5-year survival rate of <20%.9 Due to its high mortality rate, a clinical model for predicting the prognosis of patients with advanced EC is necessary. Although the International Federation of Gynecology and Obstetrics (FIGO) staging system has been widely used to predict the survival of patients with EC, this approach still suffers from several limitations.10
A nomogram is a simple visualisation tool used by oncologists to predict and quantify patient survival based on multiple variables. Nomograms have been used for patients with EC,11 and Yang et al published a nomogram for patients with stage IIIC EC following surgery.12 However, there is no specific prognostic prediction for patients with advanced EC following surgery.
The traditional statistical strategy for EC-adopted variables was significant only on univariate analysis, which led to model overfitting with generally poor results.13 Certain advanced statistical methodologies may, however, minimise this limitation. These include the best subset regression (BSR), forward stepwise regression (FSR), and least absolute shrinkage and selection operator (LASSO) approaches.14–16 In this study, we aimed to establish an effective and non-invasive nomogram to predict cancer-specific survival (CSS) in advanced EC following surgery, incorporating advanced statistical methodologies.
Methods
Data sources and patient selection
The Surveillance, Epidemiology, and End Results (SEER) Database contains cancer incidence and survival data from population-based cancer registries in the USA. EC case data with complete follow-up records were selected from the 2004–2015 SEER Database (SEER Research Plus Data, 17 Registries, November 2021 Sub (2000–2019)) using SEER*Stat V.8.4.0.1. The inclusion criteria were as follows: primary sites, C54.1–9 and C55.917; site and morphology, 8380/3 (based on the International Classification of Tumor Diseases for Oncology (ICD-O), Third Edition); histology, 8140–8389 (adenomas and adenocarcinomas); FIGO stage III/IV; and therapy and surgical treatment. The exclusion criteria were as follows: (1) undetermined survival time or survival time <1 month; (2) undetermined tumour size; (3) undetermined lymphadenectomy; (4) unknown regional node status; (5) unknown tumour grade; (6) unknown months from diagnosis to treatment; (7) unknown ethnicity and (8) unknown median household income. A flow chart of patient screening is shown in online supplemental figure 1.
Supplemental material
Data on variables, including age at diagnosis, year of diagnosis, tumour size, ethnicity, marital status, histological stage, tumour grade, FIGO stage, lymphadenectomy, regional node positivity, chemotherapy, radiation status, months from diagnosis to treatment, survival time, median household income and CSS, were collected from the SEER Database. The radiation status (with/without radiation) and chemotherapy status (with/without chemotherapy) were of two categories. Marital status was classified as unmarried (single, unmarried or living with a domestic partner), married, other (divorced, widowed or separated) or unknown. Grades were associated with each tumour. ICD-O-2 defines grade I as well differentiated, grade II as moderately differentiated, grade III as poorly differentiated and grade IV as undifferentiated. According to the SEER registry, income was examined as aggregate data based on US median income. The median household income is the median household income for the past 12 months, and it was classified into three groups: ≤54 999, 55 000–69 999 and ≥70 000. The historical stage was derived from the Collaborative Stage for 2004–2015 and divided into in situ, localised, regional, distant and unknown categories. In the localised stage, an invasive neoplasm is entirely confined to the organ of origin. In the regional stage, a neoplasm has extended (1) beyond the limits of the organ of origin directly into the surrounding organs or tissues, (2) into the regional lymph nodes via the lymphatic system, or (3) into the regional lymph nodes via a combination of extension and regional lymph nodes. In the distant stage, the neoplasm has spread to parts of the body that are remote from the primary tumour. This study categorised lymphadenectomy into two categories: with and without regional lymph node dissection. Failure to perform lymph node dissection included failure to remove or aspirate local lymph nodes, local lymph node biopsy or aspiration, or sentinel lymph node biopsy. Lymph node dissection includes the removal of an unknown number of regional lymph nodes, the removal of 1–3 regional lymph nodes, the removal of ≥4 regional lymph nodes and regional lymph node dissection with anterior lymph node biopsy.
Statistical analysis
X-tile software (Yale University, New Haven, Connecticut, USA) was used to determine the cut-off values for age at diagnosis, tumour size, positive regional nodes and risk stratification.18 Statistical analyses were conducted using R V.4.0.2 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org) in the RStudio environment, as well as with Free Statistics V.1.8 (Beijing FreeClinical Medical Technology Co). CSS was the primary endpoint of this study. The patients were randomly assigned to training and validation cohorts at a 7:3 ratio. Categorical variables are presented as frequencies and proportions. Χ2 tests were used to compare clinicopathological characteristics between the training and validation cohorts. Statistical significance was set at p<0.05. BSR, FSR and LASSO were used to select the variables. Significant prognostic factors were identified using the Cox proportional hazards model. A nomogram associated with CSS was constructed and incorporated into the known prognostic factors. The nomogram performance was validated through both training and validation, using the area under the time-dependent receiver operating characteristic curve (time-dependent AUC) to assess its discriminative abilities. Calibration curves were plotted to compare the predicted CSS with the actual CSS after 1, 3 and 5 years. The area under the curve (AUC) values ranged from 0.5 to 1.0, with 0.5 representing random variability and 1.0 representing perfect fit. AUC values g>0.7 usually indicate rational estimation. The nomogram was compared with the FIGO staging system using the net reclassification index (NRI) and integrated discrimination improvement (IDI). NRI and IDI can be used as alternatives to AUC for assessing the effectiveness of a new risk prediction model and for determining its effectiveness.19 20 The Kaplan-Meier method was used to compare the risk stratification of the nomogram.
Patient and public involvement
None.
Results
Patient characteristics
A total of 5445 patients with advanced EC following surgery were screened from the SEER Database according to our inclusion and exclusion criteria. They were randomly allocated into training (n=3812) and validation cohorts (n=1633) at a 7:3 ratio. Patient characteristics are shown in table 1. No statistically significant differences were found in the indicators between the two groups (all p>0.05).
The basic characteristics of patients with endometrial carcinoma in the study
Nomogram variable screening
Age, tumour size, regional node positivity and linear predictors (linear predictor=0.448×black ethnicity+0.166×other ethnicity−0.158×chemotherapy−0.706×historical stage regional−0.702×historical stage distant+0.25×grade II+0.913×(grade III–IV)−0.261×radiation+0.977×FIGO stage IV+0.471×(age at diagnosis 65–75 years)+0.881×(age at diagnosis 76–96 years)+0.263×(tumour size 36–78 mm)+0.57×(tumour size 79–790 mm)+0.317×(regional nodes positive 1–2)+0.619×(regional nodes positive 3–82)−0.132×(income $55 000–69 999)−0.195×(income ≥$70 000)–0.271) were divided into three categories using the X-tile software. The best cut-off ages were 64 and 75 years (online supplemental figure 2), the best cut-off tumour sizes were 35 mm and 78 mm (online supplemental figure 2), the best cut-off regional node positivities were 0 and 2 (online supplemental figure 2), and the best cut-off linear predictors were 0.2 and 1.2 (online supplemental figure 2).
Supplemental material
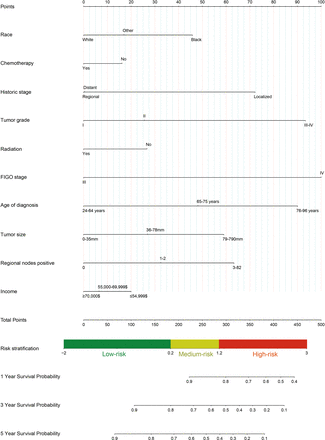
BSR, LASSO and FSR were used to select the variables. The BSR method showed great benefits for variable selection because all possible combinations of variables were calculated and the final selected combination was based on the minimum Bayesian information criterion. As is shown in online supplemental figure 3A,B, six variables (positive regional nodes, age at diagnosis, tumour size, FIGO stage, grade and ethnicity) were selected from the variables in the training cohort. Considering that the number of independent variables included in the regression equation should be ~10–15× the number of ending events, we used LASSO to select the variables. As is shown in online supplemental figure 3C,D, seven variables (positive regional nodes, age at diagnosis, tumour size, FIGO stage, grade, radiation and income) were selected from the variables in the training cohort. Furthermore, the FSR selected 10 variables (positive regional nodes, age at diagnosis, tumour size, FIGO stage, grade, ethnicity, chemotherapy, history, radiation and income) in the training cohort. As a result (online supplemental figure 4), the discrimination of the FSR was highest in the 1-year, 3-year and 5-year training cohorts, with a concordance index (C-index) of 0.808 (95% CI: 0.786 to 0.83), 0.787 (95% CI: 0.771 to 0.802) and 0.771 (95% CI: 0.756 to 0.786), respectively. Moreover, compared with LASSO and BSR (online supplemental table 1), the 1-year, 3-year and 5-year IDIs of FSR were significantly improved (FSR vs LASSO: 0.006, 0.004 and 0.003, respectively, all p<0.05; FSR vs BSR: 0.013, 0.012 and 0.011, respectively, all p<0.05). Therefore, the nomogram obtained from the FSR was optimal (figure 1). These 10 variables were obtained from the FSR using multivariate Cox analysis due to their optimal performance for predicting CSS in patients with advanced EC following surgery. The results showed that ethnicity, chemotherapy, historical stage, grade, radiation, FIGO stage, age at diagnosis, tumour size, positive regional nodes and income were independent prognostic factors in this patient group (table 2). A nomogram for predicting 1-year, 3-year and 5-year CSS was constructed based on these 10 key factors (figure 1).
Supplemental material
Supplemental material
Supplemental material
Univariate and multivariable Cox regression analyses of cancer-specific survival
Nomograms for predicting 1-year, 3-year and 5-year cancer-specific survival. Other: American Indian/Alaska Native, Asian/Pacific Islander. FIGO, International Federation of Gynecology and Obstetrics.
Nomogram construction and performance
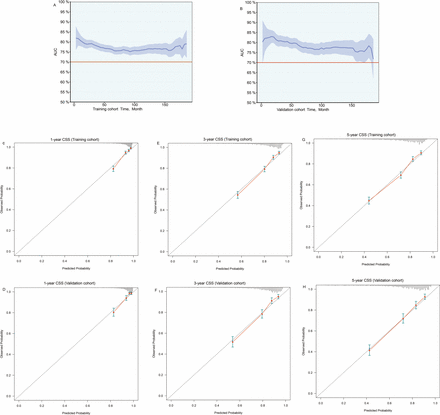
As shown in figure 1, we developed a nomogram based on FSR to predict 1-year, 3-year and 5-year CSS rates. According to the training and validation cohort data, the C-index values were 0.7324 (95% CI=0.7181 to 0.7468) and 0.7511 (95% CI=0.7301 to 0.7722), respectively. According to figure 2A,B, the AUC for the prediction of CSS within 5 years was >0.7 in both the training and validation cohorts, indicating favourable discrimination. Figure 2C,E and G shows the calibration curves of the 1‐year, 3-year and 5‐year CSS for advanced EC following surgery in the training cohort. Figure 2D,F and H shows the calibration curves of the 1‐year, 3-year and 5‐year CSS for advanced EC following surgery in the validation cohort. The dashed black line indicates the ideal reference line, where the predicted probabilities matched the observed survival rates. The red dots represent the performance of the nomogram. The closer the solid red line is to the black dashed line, the more accurately the model predicted survival. As is shown in figure 2C–H, the calibration curves of the nomogram showed high concordance between the predicted and observed survival probabilities.
Time‐dependent AUC and calibration curves of the nomogram. (A,B) Time‐dependent AUC of using the nomogram to predict cancer-specific survival (CSS) probability within 5 years in the training and validation cohorts. The red line represents AUC=0.7, which is considered ideal. (C, E and G) Calibration curves of 1-year, 3-year and 5‐year CSS for advanced EC post-surgery in the training cohort. (D, F and H) Calibration curves of 1-year, 3-year and 5‐year CSS for advanced EC post-surgery in the validation cohort. The black dashed line indicates the ideal reference line where predicted probabilities match the observed survival rates. The red dots represent the performance of the nomogram. The closer the solid red line is to the black dashed line, the more accurately the model predicts survival. EC, endometrial carcinoma; time-dependent AUC, area under the time‐dependent receiver operating characteristic curve.
Comparative clinical value of the nomogram and FIGO stage
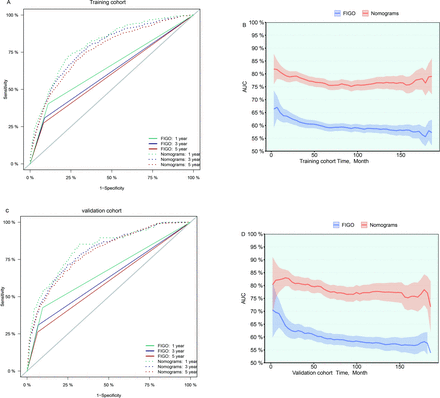
The accuracies of the nomogram and FIGO stage were compared based on changes in the receiver operating characteristic curves and time-dependent AUCs (figure 3). Compared with the FIGO stage (online supplemental table 2), the 1-year, 3-year and 5-year IDI of the nomogram was significantly greater (nomogram vs FIGO stage: 0.062, 0.099 and 0.112, respectively). Moreover, compared with the FIGO stage (online supplemental table 2), the 1-year, 3-year and 5-year NRI of the nomogram was significantly greater (nomogram vs FIGO stage: 0.364, 0.354 and 0.337, respectively). According to these results, the nomogram predicted the prognosis more accurately than the FIGO stage.
Supplemental material
Comparison of the accuracy of the nomograms and FIGO stage based on changes in the receiver operating characteristic curves and the time-dependent AUC. FIGO, International Federation of Gynecology and Obstetrics; time-dependent AUC, area under the time-dependent receiver operating characteristic curve.
Assessment of the risk of advanced EC following surgery
In addition to the nomogram, we developed a risk stratification system based on the linear predictor cut-off value for each patient in the training cohort. The patients were divided into three groups according to their linear predictors: low risk (≤0.2), intermediate risk (0.21–1.2) and high risk (>1.2). There was a significant difference in CSS between the low-risk, medium-risk and high-risk groups according to our Kaplan-Meier analysis (all p<0.05, online supplemental figure 5). Furthermore, according to the nomogram, a total score of ≤185 indicated low risk, 185≤285 indicated medium risk and >285 indicated high risk. These results show that the nomogram had excellent risk-stratification capabilities.
Supplemental material
Discussion
In this study, we used actual information from patients with advanced EC following surgery. We also developed a prognostic nomogram and risk stratification system using data from the SEER Database. The nomogram produced excellent internal and external results, as shown by calibration, C‐index and receiver operating characteristic curves.
Few studies have focused on predicting postoperative CSS in patients with advanced EC. This study focused on postoperative CSS in patients with stage III–IV cancer for two key reasons. First, advanced EC has high prognostic heterogeneity and a poor survival rate, with a 5-year survival rate of 20–60% (although different patients have different prognoses). Due to the lack of a reliable model to predict survival in patients with advanced EC following surgery, individualised clinical management and surveillance can be challenging. Second, patients with advanced EC have significantly higher incidence and mortality rates following surgery, leading to confounding bias in prognostic indicators.
EC is usually treated surgically, and postoperative treatment depends on risk factors such as age, tumour stage, myometrial infiltration depth and histological grade.21 22 In this study, a prognostic model after the surgical treatment of advanced EC was constructed based on 10 variables (ethnicity, chemotherapy, historical stage, tumour grade, radiation therapy, FIGO stage, age at diagnosis, tumour size, positive regional nodes and median household income) screened using FSR. The scores were calculated for each item based on the subtype of each independent prognostic factor. The total score was calculated using scores corresponding to the independent prognostic factors. Each subgroup variable was assigned a score from 0 to 100 according to its contribution. All enrolled variables were added to generate a total score on the bottom scale, which was then converted to predict CSS. CSS at 1, 3 and 5 years was determined by drawing a vertical line on the total score scale, with higher scores indicating a worse prognosis. According to the nomogram, the FIGO stage plays the largest role in prognosis, followed by tumour grade and age at diagnosis.
Cancer grade, histological subtype, tumour size, LVSI, lymph node status and cervical involvement are vital prognostic factors in patients with EC.23 In this study, tumour grade, tumour size and lymph node status were important prognostic factors following surgical treatment for advanced EC. Tumour grade has also been shown to be a prognostic factor in EC,24 and our nomogram indicates that poorly differentiated or undifferentiated tumours have poor prognoses. Conflicting results have been reported concerning the impact of tumour size on survival outcomes. Preoperative ultrasound tumour size was apparently not a prognostic factor for death from any cause in women with EC.25 However, tumour size was an independent prognostic factor for recurrence alone26 27 and for recurrence and death due to EC.28 Lymph node metastasis further contributes to poor prognosis in patients with EC; however, there is no consensus on the value and extent of lymph node dissection.29 In this study, we found that positive lymph nodes could affect the prognosis of surgical treatment for advanced EC, consistent with the findings of previous studies. However, this study did not reflect whether lymph node dissection was beneficial. This may be related to the fact that the population selected in this study underwent lymph node dissection (98.7%), which was not comparable. Compared with women ≥65 years, women <65 years had a significant survival advantage, as indicated by previous studies.30
Using advanced EC after surgery as a dataset, this study examined factors that could be included in prognostic nomograms. Nomograms combine multiple factors, including demographic and clinicopathological characteristics, into quantitative models that provide better predictions than FIGO staging.31 32 FIGO staging has traditionally been used to predict the prognosis of women with EC. Staging using this system is closely associated with CSS. However, patients at the same stage have different prognoses. FIGO staging does not consider factors such as age, radiation status and income, thus resulting in its prognostic heterogeneity. Therefore, we compared nomograms that included more variables. Nomograms generally have better predictive powers than FIGO staging alone due to their positive NRI and IDI scores.
Based on their total nomogram scores, the patients were classified into low-risk, intermediate-risk and high-risk groups. Significant differences were found in CSS among the three risk groups based on Kaplan-Meier analysis (online supplemental figure 5). This nomogram is highly effective in identifying high-risk groups owing to its poor prognosis. Patients with a total score greater than 285 should receive special attention.
To investigate the potential utility of the nomogram in clinical practice, we analysed data from the SEER Database by using a large sample of data representing different population regions. We followed the recommendations of the Transparent Reporting of Individual Prognosis or Diagnosis Multivariate Predictive Model statement.33 Bootstrapping and cross-validation methods were used to calculate the calibration curves, time-dependent AUCs and C-index. These positive results show that our nomogram may be useful for assessing the prognosis of patients with advanced EC after surgery.
Although the nomogram performed well, this study had some key limitations. Carbohydrate antigen 125 (CA125) is a tumour marker whose levels are often elevated in patients with malignant tumours such as ovarian epithelial, fallopian tube and EC, as well as in those with lung and gastrointestinal adenocarcinomas. In the clinical diagnosis and treatment of EC, CA125 levels are often used to monitor disease changes, evaluate treatment effects and predict prognosis.34 Studies have shown that CA125 is an important variable in the prognostic prediction model of EC and can significantly improve its accuracy.35 Human epididymis protein 4 (HE4) is an acidic whey protein first identified in the epithelium of the distal epididymis.36 It is expressed in the epithelia of several tissues, including the female reproductive tract, and is overexpressed in several cancers.37 HE4 is strongly associated with survival in patients with EC.38 ECs have traditionally been classified into two subtypes (1 and 2) based on their histopathological characteristics.2 However, this classification system lacks reproducibility and yields heterogeneous molecular groups that hamper the advancement and implementation of precision medicine.39 40 It is, therefore, being gradually replaced by a clearly defined system based on molecular phenotypes.41 The TCGA approach results in the molecular stratification of ECs into four distinct molecular groups: DNA polymerase epsilon ultra-mutated classification, which portends a good prognosis; microsatellite instability hypermutated (intermediate prognosis); copy number-low; and copy number-high (which includes p53 mutations and carries the worst prognosis).41 The European Society for Medical Oncology 2022 recommends that molecular staging testing should be performed for all ECs, but POLE testing can be omitted for low-risk patients when conditions are limited. However, MMR and p53 testing should still be performed to identify patients with hereditary EC or high-risk factors.42 LVSI has a prognostic value in patients with EC independent of TCGA signature, age and adjuvant treatment, increasing the risk of death from any cause.43 Since data on CA125, HE4, molecular typing, LVSI, hormonal therapy or immunotherapy were not published in SEER 2004–2015, these variables were not assessed in this study. In addition, the chemotherapy and radiotherapy information in the SEER Database can only be obtained by signing certain legal agreements that appeared unavailable at the time. As a result, we were unable to study the relationship between chemotherapy, radiotherapy, targeted therapy and EC prognosis. Moreover, the study cases derived from the US SEER Database were non-representative of regions outside the USA. Finally, although the predictive performance of the nomogram was evaluated thoroughly using internal data, validation using different external data sources is warranted, and further investigation is recommended.
Conclusions
Our nomogram is more accurate, has better clinical utility and provides better prognostic predictions than FIGO staging for patients with advanced EC after surgery. However, the predictive performance of the nomogram was evaluated using internal data only. Therefore, using different data sources for external validation is warranted, and further investigation is recommended.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request. Based on the SEER website (https://www cancer.gov/policies/accessibility), the National Cancer Institute (NCI) provides access to all individuals seeking information on http://www.cancer.gov. The NCI SEER Database is publicly available. Data supporting the findings of this study are available from the corresponding author upon request.
Ethics statements
Patient consent for publication
Ethics approval
This study used data from the SEER Database, approved by the National Institutes of Health Ethics Program. Access request has been approved for the SEER Research Database. Ethics approval was not required for the present analysis.
Acknowledgments
We thank the SEER Project for providing access to their database and the Free Statistics software V.1.8, provided by Beijing FreeClinical Medical Technology Co. We gratefully thank Dr Jie Liu of the Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital, for his contribution to statistical support, study design consultations and comments regarding the manuscript.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors CZ—study conception, data collection, data analysis, interpretation, drafting, critical revision and final approval of the article. RM, XW and DF—data collection. YN, FF and PZ—data analysis. ZZ and XL—study conception. WC and XX—critical revision and final approval of the article. CZ is the guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.