Article Text
Abstract
Introduction Advanced chronic liver disease (ACLD) is a major cause of death for people with HIV (PWH). While viral hepatitis coinfections are largely responsible for this trend, metabolic dysfunction-associated steatotic liver disease (MASLD) is an emerging concern for PWH. We aimed to assess the contribution of MASLD to incident ACLD in PWH.
Methods and analysis This multicentre prospective observational cohort study will enrol 968 consecutive HIV monoinfected patients from four Canadian sites, excluding subjects with alcohol abuse, liver disease other than MASLD, or ACLD at baseline. Participants will be followed annually for 4 years by clinical evaluation, questionnaires, laboratory testing and Fibroscan to measure liver stiffness measurement (LSM) and controlled attenuation parameter (CAP). The primary outcome will be incidence of ACLD, defined as LSM>10 kPa, by MASLD status, defined as CAP≥285 dB/m with at least one metabolic abnormality, and to develop a score to classify PWH according to their risk of ACLD. Secondary outcomes will include health-related quality of life (HRQoL) and healthcare resource usage. Kaplan-Meier survival method and Cox proportional hazards regression will calculate the incidence and predictors of ACLD, respectively. Propensity score methods and marginal structural models will account for time-varying exposures. We will split the cohort into a training set (to develop the risk score) and a validation set (for validation of the score). HRQoL scores and healthcare resource usage will be compared by MASLD status using generalised linear mixed effects model.
Ethics and dissemination This protocol has been approved by the ethics committees of all participating institutions. Written informed consent will be obtained from all study participants. The results of this study will be shared through scientific publications and public presentations to advocate for the inclusion of PWH in clinical trials of MASLD-targeted therapies and case-finding of ACLD in PWH.
- HIV & AIDS
- Hepatology
- Quality of Life
- Patient-Centered Care
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This multicentre, prospective study will enrol a large number of consecutive people with HIV (PWH) monoinfection at four different Canadian sites.
By dividing the cohort into a training and validation set, we will be able to develop a clinically relevant risk score to stratify PWH according to increased risk of advanced chronic liver disease (ACLD).
Thanks to the significant involvement of community allies in the development of the protocol, the study will also focus on patient-centred outcomes and quality of life.
As liver biopsy is not feasible for a large cohort study, ACLD will be assessed by transient elastography, a well-validated, non-invasive, cost-effective diagnostic tool that will help broaden the generalisability of the study and reduce selection bias.
Despite being underpowered to tease out the effect of single antiretroviral therapy (ART) drugs, we will conduct sensitivity analyses to account for cumulative ART exposure and by class.
Introduction
With 62 790 people affected in Canada and more than 38 million worldwide, the HIV continues to be a major public health issue.1 2 Antiretroviral therapy (ART) has changed the prognosis of HIV infection, with a dramatic reduction in AIDS-related deaths.3 However, as people with HIV (PWH) age, the burden of non-AIDS related mortality rises.4 Liver disease is one of the leading causes of non-AIDS related mortality.5 6 The recently proposed term advanced chronic liver disease (ACLD) reflects the continuum of advanced liver fibrosis and cirrhosis, emphasising that portal hypertension, responsible for the majority of end-stage liver complications, may occur before a formal anatomical diagnosis of liver cirrhosis.7 Liver fibrosis can be measured non-invasively using transient elastography by Fibroscan (Echosens, Paris, France), providing a safe and cost-effective identification of asymptomatic patients with ACLD at risk of developing hepatic decompensation and all-cause mortality.8 Although liver biopsy remains the gold standard, according to the Baveno consensus liver stiffness measurement (LSM) by transient elastography is sufficient to suspect ACLD.9 A variety of factors, including coinfection with hepatitis C (HCV) and hepatitis B (HBV) viruses, the high frequency of alcohol use, the hepatotoxicity of ART and metabolic dysfunction-associated steatotic liver disease (MASLD), contribute to ACLD in PWH.10 HCV infection remains the leading cause of liver-related death in PWH, but the availability of highly effective new direct-acting antivirals has dwarfed the mortality trend.11 Conversely, MASLD-related deaths in PWH are worryingly increasing, paralleling the raising prevalence of metabolic syndrome and other aging-related conditions.11 In June 2023, an international consensus panel introduced steatotic liver disease (SLD) as an umbrella term encompassing the various etiologies of hepatic steatosis.12 MASLD, formerly known as non-alcoholic fatty liver disease, is defined as evidence of hepatic steatosis with at least one cardiometabolic risk factor, in the absence of excessive alcohol intake or other known causes of SLD.12 It encompasses a range of conditions, from simple steatosis to metabolic dysfunction-associated steatohepatitis, eventually evolving to liver fibrosis and ACLD, ultimately leading to liver failure and hepatocellular carcinoma (HCC).13 14 Affecting approximately 30% of the world’s population, MASLD is the most common liver disease globally and plays a central role in the epidemic of ACLD.15 MASLD is projected to become the leading indication for liver transplantation in North America in the next 10 years.16 Beyond liver disease, MASLD is emerging as a multisystem disease affecting extra-hepatic organs and a risk factor for all-cause mortality.17 Furthermore, patients with MASLD often experience a range of mental, emotional and social problems, resulting in impaired health-related quality of life (HRQoL).18 As a result, patients with MASLD consume large amounts of healthcare resources.19
MASLD is more common and severe in PWH compared with the general population.20 Our cross-sectional data found a prevalence of MASLD up to 48%, while ACLD seems four times more prevalent than in the general population.21 22 These findings are consistent with a recent meta-analysis in PWH reporting a prevalence of MASLD on imaging studies at 34%, and a prevalence of biopsy-proven significant liver fibrosis at 23%.23 This reflects the complex and multifaceted pathogenesis of MASLD in PWH, with highly prevalent classical metabolic risk factors and unique HIV determinants, such as immune activation, virus-induced direct effect, systemic chronic inflammation and life-long exposure to ART.20 Therefore, the European AIDS Clinical Society guidelines recommend the use of transient elastography or serum fibrosis biomarkers for case-finding of liver fibrosis and ACLD in PWH without viral hepatitis coinfection and with metabolic abnormalities, or elevation of liver transaminases or any exposure to dideoxynucleoside drugs (d-drugs) such as stavudine and didanosine.24 Nevertheless, hepatological societies still do not acknowledge HIV infection as a high-risk condition for MASLD.25 PWH are excluded from global clinical trials of MASLD-targeted therapies.26 Thus, there is a need for further high-quality evidence on the role of MASLD in the epidemic of ACLD in PWH mono-infection. In addition, to better allocate healthcare resources, it is clinically relevant to identify patients at high risk for progression to ACLD and thus develop an ad hoc risk score, incorporating HIV-related features. Finally, little is known about the impact of MASLD on the HRQoL and the use of healthcare resources in PWH.
Study objectives
Primary objectives
To determine the risk of incident ACLD by MASLD status (presence/absence) and by MASLD severity as determined by LSM with controlled attenuation parameter (CAP) in PWH.
To develop a risk score to classify PWH into increasing risk categories for development of ACLD based on identified predictors.
Secondary objectives
To determine the effect of MASLD on HRQoL in PWH.
To determine the effect of MASLD on healthcare resource usage in PWH.
To establish a tissue bank of plasma and serum from 150 participants as a resource for future studies of MASLD, to better investigate correlations of additional known and unknown parameters with ACLD.
Methods and analysis
Study design
This study is a multicentre, prospective, observational cohort of PWH (LIVEr disease in HIV (LIVEHIV) Cohort study). Nine hundred and sixty-eight participants will be recruited from four sites in Canada including (1) the McGill University Health Centre (MUHC) (Montreal), (2) the Ottawa General Hospital, (3) the Toronto General Hospital and (4) the Oak Tree Clinic (Vancouver). These sites were selected because of the large number of diverse PWH they follow, the well-established research infrastructure with availability of LSM with CAP, and their history of successful enrolment in Canadian HIV Trials Network (CTN) studies.
Participants
Inclusion criteria
(1) Age >18 years old; (2) historical HIV seropositive (ELISA with Western blot confirmation); (3) able to provide informed consent, in French or English.
Exclusion criteria
(1) Presence of the outcome at baseline (ACLD); (2) liver decompensation; (3) contraindications to transient elastography (pregnancy or pacemaker); (4) unreliable LSM (interquartile range [IQR]>30%, less than 10 validated measures); (5) coinfection with HCV or HBV; (6) significant alcohol intake (Alcohol Use and Disorders Identification Test (AUDIT-C) score >4 in men and >3 in women27); (7) autoimmune liver or cholestatic disease, haemochromatosis, Wilson disease, alpha-1-antitripsin deficiency; (8) pregnancy.
All PWH meeting eligibility criteria will be approached for participation consecutively, independently of known risk factors for the primary exposure (MASLD) and outcome (ACLD).
Participant timeline
Participants will be followed on yearly basis after baseline visit for 4 years (table 1). Participants will undergo an initial evaluation (baseline) followed by visits every 12 months (±2 months). In case of any complication during the course of study participation, such as liver decompensation, end-stage liver disease or LSM≥15 kPa, the follow-up visit will be changed to twice a year for 4 years and patients will undergo surveillance for HCC according to each site’s protocol for the management of patients with ACLD, consisting of ultrasound and serum alpha-fetoprotein, as well as consideration for esophagogastoduodenoscopy following the Baveno VII consensus criteria.9 27 HIV care providers will be notified of the finding of any complication occurred during study participation.
Schedule of visits and procedures
Outcome measures
Primary outcome
The primary outcome will be the development of ACLD, defined as LSM>10 kPa, confirmed by a second transient elastography 1 week later. The main exposure will be MASLD, defined according to the recent multi-society Delphi consensus statement, as the presence of hepatic steatosis, defined as CAP>285 dB/m, plus at least one out of five cardiometabolic criteria: (1) overweight or waist circumference >94 cm for man and >80 cm for women; (2) fasting serum glucose ≥5.6 mmol/L or 2-hour postload glucose levels ≥7.8 mmol/L or haemoglobin glycosylated ≥5.7% or type 2 diabetes or treatment for type 2 diabetes; (3) blood pressure ≥130/85 mm Hg or specific antihypertensive drug treatment; (4) plasma triglycerides ≥1.70 mmol/L or lipid lowering treatment; (5) plasma high-density lipoprotein-cholesterol ≤1.0 mmol/L in men and ≤1.3 mmol/L in women, or lipid lowering treatmen and no other discernible causes of SLD.12 Severe MASLD is defined as CAP>300 dB/m.28
Secondary outcomes
HRQoL assessed by patient-reported outcome measures: Short Form-36 (SF-36) and Chronic Liver Disease Questionnaire (CLDQ).
Healthcare resource usage measured by self-reported questionnaires and linkage to provincial linked data.
Development of end-stage liver disease and cardiovascular disease.
Sample size
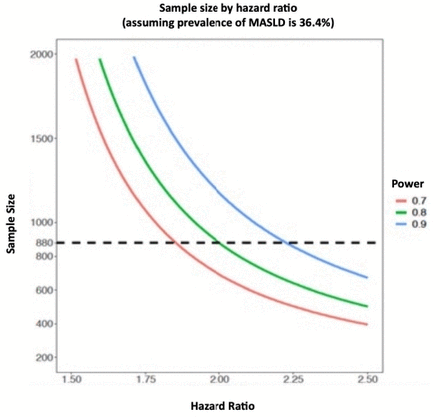
We based the sample size calculation on a conservative prevalence of MASLD in PWH of 36.4% and our published rate of progression to significant liver fibrosis (stage 2) of 12.7 (95% CI 9.5 to 17.1) per 100 person-years (PY) over 4-year follow-up.22 29 We expect a conservative incidence rate of ACLD (stages 3 and 4) of 3.0 (95% CI 1.1 to 5.5) vs 1.2 per 100 PY (95% CI 0.4 to 3.1) in patients with and without MASLD, respectively. Thus, the overall event rate would be 11% and 5% for ACLD over 4 years in patients with vs without MASLD, respectively. We require a sample size of 880 with 4-year follow-up to achieve 80% power to detect a hazard raio (HR) of 2.0 with a type I error rate of 5%. We will increase our sample size to 968 to account for 10% potential dropouts, loss to follow-up and Fibroscan failure or unreliable measurement (figure 1).
Sample size estimation. MASLD, metabolic dysfunction-associated steatotic liver disease.
Recruitment
Patients will be informed about the study either during routine HIV clinic follow-up visits or during transient elastography visits, and through established recruitment strategies by our community partners and the CTN via websites, email and social media platforms. All PWH meeting the eligibility criteria will be approached for participation. To broaden generalisability, anyone who meets the eligibility criteria may participate in the study if they can come to the enrolment sites for the study visits. Potentially eligible candidates can be notified by following the instructions provided on the CTN website.30 Participants will be compensated $25 per study visit.
Clinical, instrumental and laboratory evaluations
Clinical evaluations
Once a participant has been identified for screening, the details of the study are carefully discussed with them. The participant will be asked to read and sign the approved informed consent form prior to any assessments being performed. Clinical assessment will include measurements of body mass index (BMI), waist circumference and hip circumference to calculate waist-to-hip ratio and blood pressure. Clinical charts will be reviewed to collect information about sociodemographic characteristics, medical history (comorbidities and concomitant medications), HIV-related variables (date of HIV diagnosis, date of initiation of ART, CD4 count nadir, history of ART), development of end-stage liver disease (oesophageal varices, hepatic encephalopathy, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, HCC, liver transplantation or liver-related death) and development of cardiovascular disease (myocardial infarction, angina, stroke or peripheral arterial disease).
Transient elastography and serum fibrosis biomarkers
Transient elastography examination will be performed on a 6-hour fasting participant who has been informed to avoid strenuous exercise prior to testing. Four certified operators (>100 examinations before the study), one per site, blinded to clinical and biological data, will perform 10 valid acquisitions of LSM and then the software of Fibroscan will calculate the median value.21 The standard M probe will be used in all patients. The XL probe will be used in patients with BMI>30 kg/m2 and in case of failure of Fibroscan examination by the M probe. The following manufacturer recommendations will be applied to define the result of Fibroscan as reliable: 10 validated measures, IQR<30% of the median.31 Presence of significant liver fibrosis and ACLD will be defined as LSM>7.1 kPa and LSM>10 kPa, respectively.9 32 CAP measurement will be performed simultaneously to assess liver steatosis.33 The following decisional cut-offs will be applied: no MASLD (CAP<285 dB/m), mild MASLD (CAP 285–300 dB/m) and severe MASLD (CAP>300 dB/m).28 We will also compute the serum fibrosis biomarkers AST-to-Platelet Ratio index and fibrosis-4 index, as previously described in PWH.34
Questionnaires
Self-assessment questionnaires will be completed by participants at each visit to collect:
Medical, sociodemographic and lifestyle information: AUDIT-C35; eating habits assessed by the Food Frequency Questionnaire-Short Form36; physical activity assessed by the International Physical Activity Questionnaire-Short Form37 38; substance use behaviour; smoking habits; tea and coffee use; HIV adherence treatment scale.
Patient reported outcomes and HRQoL: CLDQ and SF-36. The CLDQ is a validated liver disease specific quality of life index consisting of 29 items divided into six subscales and correlated with the severity of liver disease.39 Scores on the six subscales range from 1 to 7. An overall CLDQ score is then calculated (range=1–7). Higher scores on each CLDQ scale reflect better HRQoL.40 The SF-36 consists of 36 questions that make up eight subscales.41 Within each dimension, 0 is the worst and 100 is the best possible score. An overall SF-36 score is then calculated, with a score greater or less than 50 representing better or poorer health than the general population, respectively.41
Healthcare resource usage: report of number of visits to family medicine doctors, specialists, emergency rooms.
Participants who require help with reading and writing will be assisted by the study coordinators.
Laboratory test review
The results of the blood tests obtained for clinical evaluations at each visit as per routine standard of care will be reviewed. Routine haematology, chemistry and serological evaluations will include: liver function tests (alanine transaminase, aspartate aminotransferase, gamma-glutamyl transferase, alkaline phosphatase, total bilirubin, albumin, coagulation profile); complete blood count; biochemistry (fasting lipid profile, glucose, insulin and glycosylated haemoglobin, renal function tests); autoimmune markers (autoantibodies, immunoglobulins); serum ferritin and transferrin saturation; hepatitis A, B and C serology; HIV-related variables (plasma HIV RNA, absolute and relative CD4, nadir CD4, CD8 T-cell counts, CD4/CD8 ratio).
Biobanking samples
Due to infrastructure constraints, biobanking samples will take place at the MUHC. Only plasma and serum from blood will be collected, and it will be stored for as long as the bank has a scientific interest to the community and the research team can ensure its management. To avoid multiple blood draws and visits, blood collection will be scheduled during routine clinical blood draw.
Statistical analyses
Primary analysis
Specific aim 1: determine the incidence of ACLD according to MASLD status
The cumulative incidence of ACLD (primary outcome) will be calculated using the Kaplan-Meier survival method, comparing individuals with versus without MASLD, as assigned at baseline. A propensity score method will be applied to account for imbalances in the covariate distributions between participants with and without MASLD at baseline using inverse probability weighting (IPW).42 43 Next, to account for the time-varying nature of the exposure and confounders, we will use a multinomial logistic model using time-varying covariates to calculate IPW for the categorical exposure (MASLD) to represent escalating severity of the disease. Marginal structural models with stabilised IPW weights will be calculated at each follow-up visit. We will use an absolute standardised mean difference of 0.1 as a cut-off. We hypothesise a dose–response of our exposure, meaning increased liver fat increases the risk of ACLD. Liver fat will be evaluated in three levels: no MASLD (CAP<285 dB/m); mild MASLD (CAP 285–300 dB/m) and severe MASLD (CAP>300 dB/m). To account for baseline liver fibrosis severity measured by transient elastography, we will also conduct a stratified analysis of the incidence of ACLD according to the baseline presence of significant liver fibrosis.
Specific aim 2: develop a risk score to predict development of ACLD
The full cohort will be divided into a training set (to develop the risk score) and a validation set to internally validate the score. Associations of predictors with ACLD will be analysed using Cox proportional hazards regression. Weights will be assigned for each predictor and the risk score will be computed as a linear combination of the weighted predictors.44 45 The 5-year risk of ACLD will be calculated by inserting the individual risk score into the survivor function from the proportional hazards model:  where SM=survivor function estimate at 5 years and at means of all predictors, RSi=individual risk score estimated as the linear combination of weighted predictors and RSm=risk score estimated at means of all predictors. Departure from the proportional hazards assumption will be evaluated for all predictors based on Schoenfeld residuals. Apart from the full model defined a priori, we will fit a reduced model consisting of those predictors that were significantly related to ACLD in the full model and will test for possible interactions between various predictor variables (particularly by biological sex).46 Model performance will be evaluated by means of discrimination and calibration.47 Discrimination is described by the c index for survival analysis, which quantifies the model’s ability to separate persons with longer event-free survival from those with shorter event-free survival. In addition, we will compute the continuous net reclassification improvement (NRI) to compare the discriminatory ability of the different models. NRI values above 0.6 are considered strong, and values below 0.2, weak. For the category-based NRI, we will use three clinically relevant risk categories: low risk, <10%; medium risk, >10%–25% and high risk, >25%.48 The categories are determined based on estimates of mortality from liver disease in those with chronic HCV infection from published reports, as well as opinions of knowledgeable hepatologists and clinicians.49 50 Calibration measures how well predicted probabilities agree with observed risks. We will present plots comparing observed proportions of events (from the validation sample) against average predicted probabilities across tenths of predicted risk. We will calculate sensitivity, specificity, positive predictive value and negative predictive value for a range of potential cut-off points to define high-risk individuals. To find the optimal cut-off point, we will use the Youden index (J), defined as J=sensitivity+specificity–1.51 It allows finding the threshold for which sensitivity and specificity are maximised.
where SM=survivor function estimate at 5 years and at means of all predictors, RSi=individual risk score estimated as the linear combination of weighted predictors and RSm=risk score estimated at means of all predictors. Departure from the proportional hazards assumption will be evaluated for all predictors based on Schoenfeld residuals. Apart from the full model defined a priori, we will fit a reduced model consisting of those predictors that were significantly related to ACLD in the full model and will test for possible interactions between various predictor variables (particularly by biological sex).46 Model performance will be evaluated by means of discrimination and calibration.47 Discrimination is described by the c index for survival analysis, which quantifies the model’s ability to separate persons with longer event-free survival from those with shorter event-free survival. In addition, we will compute the continuous net reclassification improvement (NRI) to compare the discriminatory ability of the different models. NRI values above 0.6 are considered strong, and values below 0.2, weak. For the category-based NRI, we will use three clinically relevant risk categories: low risk, <10%; medium risk, >10%–25% and high risk, >25%.48 The categories are determined based on estimates of mortality from liver disease in those with chronic HCV infection from published reports, as well as opinions of knowledgeable hepatologists and clinicians.49 50 Calibration measures how well predicted probabilities agree with observed risks. We will present plots comparing observed proportions of events (from the validation sample) against average predicted probabilities across tenths of predicted risk. We will calculate sensitivity, specificity, positive predictive value and negative predictive value for a range of potential cut-off points to define high-risk individuals. To find the optimal cut-off point, we will use the Youden index (J), defined as J=sensitivity+specificity–1.51 It allows finding the threshold for which sensitivity and specificity are maximised.
Secondary analysis
HRQoL scores and healthcare resource usage will be compared by MASLD status using generalised linear mixed effects model. Negative binomial regression analyses will also be used to ascertain how diagnosis relates to the HRQoL domains and to healthcare resource usage, controlling for sex, ethnicity, BMI, Fibroscan score, diabetes and HIV-related variables (ART regimens, HIV viral load, CD4 cell count, nadir CD4).
Ethics and dissemination
Ethics
This study will be conducted in accordance with the Canadian Tri-Council Policy Statement Version 2 and the principles in the Declaration of Helsinki. Written informed consent will be obtained from all study participants. Patients at the MUHC will be invited to participate into the biobank substudy. Participants who decline consent for biobanking study will be allowed to participate in the cohort study only. At the time of initial manuscript submission (June 2023), the protocol of the LIVEHIV Cohort has been approved by the ethics committees of all participating institutions: (1) MUHC Research Ethics Board (REB), (2) Ottawa Health Science Network REB, (3) University Health Network REB, (4) the Oak Valley Health REB. Patient enrolment for this trial began in January 2022 at the MUHC, and it will begin in July 2023 at the other sites. Both the protocol and informed consent forms were reviewed and approved by the CTN Community Advisory Committee.
Availability of data and materials
All participant-related information will be kept confidential. All records will be kept in a secure, locked location only accessible to research staff. Participants will be identified only by a coded number specific to each participant. All computerised databases will identify participants by numeric codes only and will be password protected. On request, and in the presence of the investigator or his/her representative, participant records will be made available to the study sponsor, monitoring groups representative of the study sponsor, representatives of funding groups, and applicable regulatory agencies for the purpose of verification of clinical study procedures and/or data, as is permissible by local regulations.
Dissemination
We will share research findings to the broader research communities by scientific and lay publications and through presentations at scientific conferences and community-based HIV clinics. A knowledge translation working group will systematically synthesise the findings of this study and tailor knowledge for identified stakeholders (decision makers, clinicians, PWH and their advocates).
Patient and public involvement
Our team is committed to integrated knowledge translation by involving community members and PWH in the design and interpretation of the analysis, with two of the CTN coapplicants and coauthors (CP and KM) being community member leaders in dissemination and advocacy for PWH. Community members were engaged in evaluating the research questions of this study, which were considered high priorities for PWH.
Discussion
Herein, we present the protocol for a multicentre observational cohort study designed to evaluate the role of MASLD in the development of ACLD in PWH and to develop a risk score to classify PWH according to their risk of incident ACLD. This study will also investigate the impact of MASLD on HRQoL and healthcare resource usage in PWH. The results of this study will be of immediate importance since PWH face inequities and disadvantages as they are currently excluded from global clinical trials of new MASLD-targeted therapies able to halt the progression to ACLD and from case-finding strategies in MASLD.25 26
Whether MASLD is a key player or a mere bystander of ACLD in HIV mono-infection is still a matter of debate.52 53 This knowledge gap is likely due to the complexity of factors that influence the development of both MASLD and ACLD. Indeed, many factors contribute to the complex pathogenesis of ACLD in PWH (figure 2). On one hand, metabolic disorders are increasing in PWH and contribute to the growing prevalence of MASLD.20 54 Diabetes is four times more prevalent in HIV-positive vs HIV-negative men.55 Dyslipidaemia and hypertension are also very common.4 56 In this protocol, metabolic aspects related to MASLD and ACLD are carefully considered, with markers of insulin resistance, lipid profile, anthropometric measures and concomitant antidiabetic drugs collected and scheduled for analysis. We will also develop a risk score including metabolic parameters to predict which PWH are at high risk of ACLD. On the other hand, unique risk factors may also contribute to ACLD in PWH mono-infection, who represent 86%–89% of PWH.10 First, the lifelong use of ART may result in liver fibrosis and oxidative stress in hepatocytes.57 58 Earlier nucleoside reverse transcriptase inhibitors, particularly d-drugs that are known to induce mitochondrial damage and impaired fatty acid oxidation in the hepatocytes, may have had a detrimental effect that could be realised as the population ages with HIV.59 Ritonavir-boosted protease inhibitors can lead to direct liver cell stress.60 Integrase strand transfer inhibitors (INSTI) and tenofovir alafenamide (TAF) may cause weight gain, although the metabolic and hepatic consequences of these newer agents is unknown, with one study suggesting INSTI and TAF as independent predictors of incident hepatic steatosis,61 and others reporting reduced or no changes in liver fat after INSTI switching.62–64 Longer observational studies are warranted to assess the effect of INSTI on the incidence of MASLD in PWH. Second, HIV viraemia from ART treatment interruptions is a risk factor for ACLD, as HIV itself may have pro-apoptotic effects on hepatocytes.57 Given this complexity, we may find that exposure to ART, rather than MASLD, is the major contributor to the development of ACLD in HIV mono-infected patients. Should this be the case, our findings will still provide crucial information for subsequent monitoring and screening strategies for ACLD in PWH. Our prognostic score for development of ACLD will be modelled accordingly, by weighing on exposure to ART rather than MASLD. Furthermore, our findings in Canada can serve as a model for other/different HIV realities, such as low/middle-income countries, where ART is becoming widespread, resulting in an ageing HIV-infected population and an increase in non-communicable diseases.
Hypothetical pathogenesis of advanced chronic liver disease in HIV mono-infection. ACLD, advanced chronic liver disease: ART, antiretroviral therapy; LIVEHIV, LIVEr disease in HIV; MASLD, metabolic dysfunction-associated steatotic liver disease.
Although we will make careful ascertainment of covariates to control for potential confounders, and will employ sophisticated analysis to minimise biases, some issues may nevertheless arise.
We may encounter slower recruitment rate or higher loss to follow-up than expected. In this case, we will consider including 1–2 other HIV care centres through our close link to CTN.
We acknowledge the complexity of the ART exposure, and we will not be powered to tease out the effect of single ART drugs. However, we will conduct sensitivity analysis to account for cumulative ART exposure and by class, recent treatment interruptions and d-drugs exposure. Although not the primary aim, this cohort will be able to explore the effect of ART on incident ACLD, which can be used for future studies. Special attention will be given to INSTI and TAF in the sensitivity analysis, considering the current open debate on weight gain and/or their role in MASLD development and progression to ACLD in PWH.
Our sample size will be limited to evaluate biological sex and the complexity of premenopause/postmenopause as an effect modifier; however, our cohort will remain the largest sample of women with HIV attempting to address this research question. Our data will be crucial to advocate for inclusion of women in future studies.
We will be underpowered to assess outcomes with expected lower incidence (end-stage liver diseases) or to conduct a stratified analysis by baseline fibrosis stage. However, our study can be used to power subsequent larger-scale multicentre studies. We also plan to expand the LIVEHIV Cohort to an International Cohort through the Steatohepatitis in HIV Emerging Research Network,25 26 65 with the purpose of assessing these rare outcomes, as well as validating externally the prognostic risk score.
The lack of a histological diagnosis is a limitation. However, transient elastography provides a simultaneous point-of-care assessment of liver fat and fibrosis, and has been extensively validated in MASLD66 67 and in PWH.32 33 68 Besides, non-invasive diagnostic tools are patient-friendly, cost-effective and help broaden the study generalisability and reduce selection biases. We will also compute and follow longitudinally validated serum fibrosis biomarkers.
Most participants will be recruited from tertiary hospitals, with possible over-representation of complex cases and under-representation of marginalised patients, such as indigenous people. However, we will attempt to broaden generalisability by promoting the study through community partners and the CTN via websites, email and social media.
In conclusion, our findings will demonstrate that HIV is a convergence for two epidemics, ACLD and MASLD, thereby generating evidence to advocate for equity and inclusion of PWH in global trials of new antifibrotic molecules for MASLD and case-finding of PWH at high risk of developing ACLD. Overall, we expect that our results will change clinical practice, policies and improve healthcare outcomes for PWH.
Ethics statements
Patient consent for publication
References
Footnotes
FC and SS are joint first authors.
Twitter @WesalElgretli, @DrMelanieMurray
FC and SS contributed equally.
Contributors The principal investigator of the study is GS. Site investigators of the study are MBK, CTC, SLW, NP, MCMM. Other CTN coapplicants are SS (Methodologist), EM (Methodologist), KM (Community Representatives), CP (Community Representatives). GS conceived the study, led the proposal and protocol development. SS and EM provided methodological expertise and performed sample size calculations. FC, DK, LRB, WE, BL, JC, NK, CTC, J-PR and AdP are involved in patient enrolment. FC, SS and GS wrote the first draft of the manuscript. MNK, CTC, SLW, EEMM, NP, MCMM, DK, LRB, WE, JC, BL, NK, CTC, J-PR and AdP reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding The study was funded by an operating grant from the Canadian Institute of Health Research (CIHR) and by the CIHR Canadian HIV Trials Network. WE has received PhD fellowship from the Canadian Network on Hepatitis C (CanHepC). CanHepC is funded by a joint initiative of the Canadian Institutes of Health Research (NPC-178912) and the Public Health Agency of Canada. SLW is the Speck Family Chair in emerging infectious diseases. NK and CTC are supported by a Fonds de Recherche du Québec–Santé (FRQS) Junior two career award. AdP is supported by a Senior Salary Award from FRQS. BL is supported by two career awards: a Senior Salary Award from Fonds de recherche du Québec–Santé (FRQS) (#311200) and the LE 250 (from the Québec Ministry of Health for researchers in Family Medicine), from the Quebec’s Ministry of Health for researchers in Family Medicine and holds a Canadian Institutes for Health Research, Strategy for Patient-Oriented Research Mentorship Chair in Innovative Clinical Trials for HIV Care. J-PR is the holder of the Louis Lowenstein Chair in Haematology and Oncology, McGill University. GS is supported by a Senior Salary Award from FRQS (#296306).
Competing interests BL has acted as a speaker and advisory board member for ViiV, Gilead and Merck, and received research funding from ViiV, Merck and Gilead. CTC has received research funding from Merck, Gilead and Tilray, speaker honorarium from Gilead and consultant fees from ViiV Healthcare and Moderna. She has received funding to attend conferences from Gilead and ViiV Healthcare, and cannabinoids from Tilray for use in a clinical trial. SLW has served on advisory board, and spoken at CME event for Merck, Gilead, Viiv, and has received research funds from ViiV, Gilead and Merck. JC has received funding for investigator-initiated research from ViiV Healthcare and remuneration for advisory work from Gilead Canada and ViiV Healthcare. MCMM has served on advisory boards and spoken at CME events for Merck, Gilead and Viiv, and has received research funds from Viiv. GS has acted as speaker for Merck, Gilead, Abbvie, NovoNordisk, Pfizer, served as an advisory board member for Pfizer, Merck, NovoNordisk, Gilead and Intercept and has received unrestricted research funding from Theratecnologies. FC, SS, DK, LRB, WE, EEMM, CP, KM, NP, NK, AdP, J-PR, MBK have no conflict of interests.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.