Article Text
Abstract
Introduction Adnexal torsion is a surgical emergency and its prognosis depends on the time elapsed prior to treatment. The diagnosis relies on pelvic ultrasound in which sensitivity remains low and may lead to misdiagnosis.
The primary objective is to evaluate the diagnostic performance of contrast-enhanced ultrasound for the diagnosis of adnexal torsion in women with suspected adnexal torsion. The secondary objectives are: (1) to describe the perfusion parameters of the ovaries by contrast-enhanced ultrasound, (2) to compare diagnostic performance of contrast ultrasound with bidimensional (2D) Doppler for the detection of adnexal torsion, (3) to describe the perfusion parameters of the ovarian as a function of the degree of adnexal torsion, (4) to compare perfusion parameters before and after ovarian detorsion and (5) to describe perfusion parameters of the ovarian by using MicroVascular Flow technique.
Methods and analysis This is a monocentric, prospective comparative, non-randomised, open and interventional study. We hypothesise to include 30 women: 20 positive cases compared with 10 control cases. Women are informed and recruited in the emergency ward, over a period of 36 months.
The primary endpoint is the signal intensity measurement to assess sensitivity, specificity, positive and negative predictive values of contrast-enhanced ultrasound for detection of adnexal torsion in women with suspected adnexal torsion. The presence or absence of adnexal torsion is confirmed during the surgical intervention.
Ethics and dissemination The study was approved by the French Ethics Committee, the CPP (Comité de Protection des Personnes) OUEST I on 3 July 2020 with reference number 2020T1-16. The results of this study will be published in a peer-reviewed journal and will be presented at relevant conferences.
Trial registration number ClinicalTrials.gov registry (NCT04522219); EudraCT registry (2020-000993-27).
- gynaecology
- genitourinary imaging
- ultrasound
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This is the first study in adults evaluating the interest of contrast-enhanced ultrasound for the diagnosis of adnexal torsion.
This imaging technique has clinical applicability, including in the emergency setting.
This study includes simultaneous evaluation of functional imaging techniques with and without contrast injection.
This study will not be able to assess the potential benefit in the event of false negative, without surgical management.
The sample of patients is small.
Introduction
Adnexal torsion is a surgical emergency due to the total or partial rotation of the adnexa around its vascular axis (the ovary and in rare cases the fallopian tube). Most cases occur in women of childbearing age and the delay in treatment can lead to ischaemia of the adnexa.1 This ischaemia may lead to haemorrhagic necrosis of the ovary that could impair its functionality and, consequently, lead to female infertility.2 Treatment is exclusively surgical by laparoscopy or laparotomy and must be performed as soon as possible to preserve ovarian function.3
Early diagnosis is an essential prerequisite to reduce the potential consequences of ovarian torsion. A short time frame between the occurrence of torsion and the surgical intervention allows conservative treatment without functional consequences in 90% of cases.2 Usually, this time frame is approximately 6 hours.
The clinical diagnosis is challenging since the main clinical sign is pelvic pain of sudden onset, a non-specific sign not allowing for a precise diagnosis.
To confirm the diagnosis, pelvic ultrasound with or without Doppler flow analysis is widely used.4 However, its contribution is low, with a sensitivity ranging from 46% to 73% depending on the study and recently confirmed by a meta-analysis.5 6 Other imaging techniques have been considered, such as MRI, with much greater sensitivity than ultrasound. However, the limited accessibility of MRI scanners, particularly in the context of emergency, makes it difficult to use in clinical practice.7 8
Ultrasound remains the most appropriate modality. SonoVue ultrasound contrast injection improves blood echogenicity and signal-to-noise ratio.9 This product consists of sulphur hexafluoride microbubbles acting as reflectors of the ultrasound beam with a diameter comparable to one of a red blood cell (approximately 6 µm): it is a strict intravascular contrast product.
Therefore, contrast-enhanced ultrasound technique seems perfectly suited to assess the vascularisation of the ovary and improve the diagnostic sensitivity of adnexal torsion. Its benefit has already been demonstrated in the diagnosis of testicular torsion in animals, but to date, only one study has evaluated its contribution in adnexal torsion.9 10
This study by Trinci et al, which assessed the diagnostic performance of contrast-enhanced ultrasound in 20 cases of adnexal torsion, was a retrospective study, performed on a paediatric population. The sensitivity was 94.1% and the specificity was 100%, that is, an overall accuracy of 95%.10 It confirmed the diagnostic contribution of contrast-enhanced ultrasound as a complement to conventional 2D ultrasound in the diagnosis of adnexal torsion.
Methods and analysis
Objectives
The primary objective is to evaluate the diagnostic performance of contrast-enhanced ultrasound for the diagnosis of adnexal torsion in women with suspected adnexal torsion.
The secondary objectives are:
To describe the perfusion parameters of the ovaries by contrast-enhanced ultrasound.
To compare performance diagnosis of contrast ultrasound with 2D Doppler for the detection of adnexal torsion.
To describe the perfusion parameters of the ovarian as a function of the degree of adnexal torsion.
To compare perfusion parameters before and after ovarian detorsion.
To describe perfusion parameters of the ovarian by using MicroVascular Flow technique.
Trial design
The AGATA protocol is a monocentric, prospective, comparative, non-randomised, open and interventional study.
Study population
Women will be informed and recruited in the emergency ward, over a period of 36 months. All the women suspected of adnexal torsion and with planned surgery who agree to participate in the study will be recruited, despite their medical history and previous examination results. The indication for surgery will be established by the surgeon, at his discretion, on the basis of a number of arguments in favour of adnexal torsion: sudden onset of pain, vomiting, pain not relieved by analgesics, ultrasound signs: enlarged adnexa, presence of an ovarian cyst, ovarian stromal oedema, absence of Doppler, etc. The inclusion and exclusion criteria are reported in table 1. The consent collection will be provided by the investigators (obstetricians).
Inclusion and exclusion criteria
The presence or absence of adnexal torsion was confirmed during the surgical intervention and women were classified retrospectively into two groups: ‘ovarian torsion’ (positives cases) or ‘no ovarian torsion’ (control cases).
Ultrasound acquisition
Ultrasound acquisitions will take place in three stages:
Identification of ovaries in 2D ultrasound.
Ultrasound acquisitions without injection of contrast product (MicroVascular Flow).
Ultrasound acquisitions with injection of contrast product (contrast-enhanced ultrasound).
These acquisitions will take place at different times of the medical care:
Before surgery: bilateral acquisition.
After surgery: unilateral acquisition on the affected ovary in case of confirmed torsion.
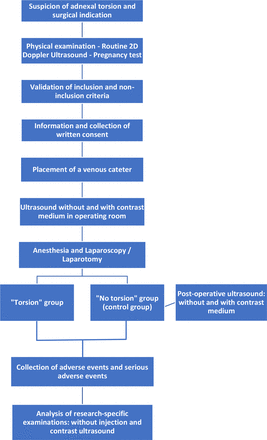
The flow chart of women participation is in figure 1.
Flow chart of women participation. 2D, bidimensional.
The ultrasound acquisition will be performed with a HERA W10 (Samsung) and with an endovaginal transducer (3–10 MHz). The ultrasound contrast product used will be SonoVue (Bracco Imaging, Italy), administered by bolus injection. A volume of 2.4 mL of contrast product will be injected per acquisition with a maximum of three injections per woman.
The contrast-enhanced acquisition will be performed in contrast mode with standardised parameters. The MicroVascular Flow acquisition will be performed in MV-Flow mode. Dynamic acquisition will be recorded during 2 min, and saved as videoclip.
Image analysis
The analysis of contrast-enhanced ultrasound will be performed with a specific offline software (VueBox, V.7.0.26, Bracco Suisse SA, Geneva, Switzerland). Region of interest will be manually drawn on the overall ovaries allowing to obtain time intensity curve for the quantitative analysis (semiquantitative perfusion indicators).
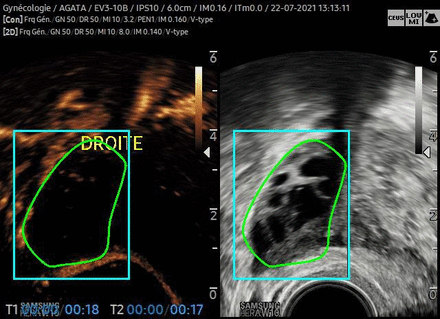
The analysis of MicroVascular Flow ultrasound will be performed on the sonographer with a specific integrated software. The region of interest will be drawn, and quantitative parameters will be extracted (figure 2).
Ultrasound acquisition in contrast-enhanced ultrasound.
Outcomes
The primary endpoint is the signal intensity measurement to assess sensitivity, specificity, positive and negative predictive values of contrast-enhanced ultrasound for detection of adnexal torsion in women with suspected adnexal torsion with realisation of ROC curves (receiver operating characteristics).
The secondary endpoints are:
Measurement of perfusion parameters of the suspected ovarian torsion and the contralateral ovary if available: signal intensity and perfusion kinetics.
Measurement of signal intensities to assess sensibility and specificity of contrast-enhanced ultrasound and 2D Doppler.
Comparison of perfusion parameters of the ovary with the degree of torsion. The degree of torsion is defined by the number of twists (number of turns around the axis) detected during the surgical procedure.
Measurement of signal intensities before and after ovarian detorsion.
Measurement of signal intensities obtained by MicroVascular Flow technique.
Participant timeline
The enrolment of women started in April 2021. The recruitment should be achieved by April 2024. The flow chart of women participation is presented in figure 1.
Premature ending of patient participation
Each person can stop participating in the research at any time and for whatever reason.
The investigator may temporarily or permanently interrupt a person’s participation in the research for any reason that has an impact on her safety or that would best serve the interests of the person who is suitable for research.
In the event of a premature ending or in the event of consent withdrawal, this shall not affect the activities carried out and the use of data obtained on the basis of informed consent before it has been withdrawn, unless the person indicates in writing that she objects to their use.
Follow-up
A clinical examination will be performed on the inpatient ward at 24 hours by an obstetric gynaecologist to look for any adverse events that have occurred since the inclusion visit.
Sample size consideration
As the research is innovative, no preliminary published figures are available to estimate a number of women to be included. Therefore, we choose to include 30 women. With an assumed distribution of 20 positive cases against 10 control cases, an accuracy of 5% and a power of 95%; this sample will lead to an area under the curve of 85% (R V.3.6.0, pROC package).
Data collection and management
The data to be collected in this study are summarised in table 2.
Data collected
An electronic case report file will be created for each woman. The women’s anonymity will be ensured by mentioning to the maximum extent possible their research code number, followed by the first letter of the last name and first name of the participant on all necessary documents or by deleting their names by appropriate means (white-out) from the copies of source documents intended to document the study.
Data from all women will be centralised and data management will be carried out by Nancy CIC-IT. Conditions for data transfer of all or part of the study database are decided by the study sponsor (Centre Hospitalier Régional Universitaire (CHRU) de Nancy) and will be the subject of a written contract. Imaging data will be transferred to Nancy CIC-IT and stored after verification, in the ArchiMed database declared to the French authority (CNIL declaration number: 1410005). This study and the collected data fall within the scope of Reference Methodology MR001.
Statistical analysis
The initial characteristics of the women at inclusion will be described in two groups. The quantitative parameters will be described by their means±SD or median and interquartile difference according to their normality, maximum and minimum and the qualitative parameters by their numbers and percentages. The normality of the distributions will be checked graphically by histograms and by the Shapiro-Wilk test.
For the main objective, construction and analysis of the ROC curve in order to obtain the signal intensity threshold (determined by the Youden Index) to use in order to estimate the sensitivity, specificity, predictive and negative predictive values of contrast-enhanced ultrasound. These parameters will be calculated with reference to the surgical intervention.
The overall alpha significance threshold is set at p<0.05 in a bilateral situation. Statistical analysis will be performed with R V.4.1.1 or superior. We will not perform an interim analysis.
Patient and public involvement
Patients and the public were not involved.
Ethics and dissemination
The study was approved by the French Ethics Committee, the CPP (Comité de Protection des Personnes) OUEST I on 3 July 2020 with reference number 2020T1-16, and the competent authority (Agence Nationale de Sécurité du Médicament et des Produits de Santé) authorised the study on 16 July 2020. The study results will be published in a peer-reviewed journal and will be presented at relevant conferences.
Quality control
Right of access to data and source documents
The CHRU de Nancy is the sponsor and is responsible for obtaining the agreement of all parties involved in the study so as to guarantee direct access to source data, source documents and reports so that the sponsor may control data quality and perform an audit.
Investigators will make available the documents and individual data strictly required for monitoring, quality control and audit of the biomedical study to persons having access to these, in accordance with the statutory and regulatory provisions in place (articles L.1121–3 and R.5121–13 of the French Public Health Code).
Any original document or object that allows the existence or accuracy of a data point or information recorded during the study to be proved is defined as a source document.
In accordance with the statutory provisions in place (articles L.1121–3 and R.5121–13 of the French Public Health Code) the persons having direct access to source data will take every precaution required to ensure the confidentiality of information relating to investigational medicinal products, studies, participants, notably concerning the identity of these, as well as the results obtained. These persons, as the investigators themselves, are subject to professional confidentiality.
During the study, or at its conclusion, data collected regarding participants that is sent to the sponsor by the investigators (or all other specialists involved) will be coded by the inclusion number of the patient in the study. At no point should the names of participants or their addresses appear unencrypted. The presentation of the data processing results can not in any case allow the direct or indirect identification of persons lending themselves to research.
The sponsor will ensure that each study participant has given her written consent for access to her personal data that is strictly required for study quality control.
The data may be transmitted to the companies in the group to which the sponsor belongs and to its contractual partners in a form which should not permit the direct or indirect identification of persons lending themselves to research.
Study monitoring
The monitoring visits (implementation, follow-up and closure) will be performed by the promoting cell of Nancy DRCI (Délégation à la Recherche Clinique et à l’Innovation – CHRU Nancy).
A Clinical Research Associate will travel regularly to the centres to perform the quality control of the study.
Depending on monitoring reports and deviations observed, the sponsor reserves the right to modify the level of monitoring initially planned.
Potential risks related to the study
The only constraint of the study for the participant is the completion of two additional ultrasound examinations, with and without contrast injection: one examination before general anaesthesia (on the two ovaries) and a second examination after the patient wakes up (only on the ovary with torsion). The time frame between the SonoVue injections for the first and second examinations will be at least 1 hour. In case of suspected reaction after the first injection, whether severe or not, the second injection will not be performed.
There are no specific medical risks for women in this study. SonoVue injection has rare known side effects, usually transient and mild.
Ethical permission
The sponsor and investigators undertake to carry out this research in accordance with the recommendations of the Helsinki Declaration and its revisions, the European Regulation (EU) n° 536/2014 from the European Parliament about clinical trials of medicines for human use, repealing European Directive 2001/20/CE, the n° 2004–806 law of 9 August 2004 about public health policy, the n° 2004–800 law of 6 August 2004 about bioethics, the No 78–17 law of 6 January 1978 relating to data processing, files and freedoms, the n° 2012–300 law of 5 March 2012 about research involving the human person, the 2016–41 law of 26 January 2016 of modernisation of our healthcare system and 2016–800 ordinance of 16 June 2016 relating to researches involving the human person and their implementing decrees.
They undertake to comply with all laws and regulations that may apply to research.
The investigators undertake to respect the protocol in all respects especially with regard to obtaining consent and the notification and follow-up of serious adverse events.
Protocol amendment
Requests of authorisation and/or opinion about substantial amendments will be addressed by sponsor to regulatory institutions.
By signing this protocol, the investigator commits to submit to the Direction of Research and Innovation the substantial amendment project and wait for authorisation and/or opinion of regulatory institutions prior to the application of amendment.
Final research report
The final report of the research will be written collaboratively by the coordinator and the biostatistician mandated for this search. This report will be submitted to each of the investigators for review. Once a consensus has been reached, the final version must be endorsed with the signature of each of the investigators and sent to the sponsor as early as possible after the effective end of the research. A report prepared according to the reference plan of the competent authority must be forwarded to the competent authority and the CPP within a year after the end of the research, understood as being the last follow-up visit of last enrolled subject. This period is abrogated to 90 days in case of premature termination of the research.
Ethics statements
Patient consent for publication
Footnotes
Contributors OM, GH and MB contributed to the conception and design of the study. RP, A-LF and CB are the coordinating investigators. AC is the study project manager. CB, RP and A-LF wrote the manuscript. CB, A-LF, RP and KG will carry out recruitment, ultrasound acquisition and will collect the data. MB is supervising data processing. GH is in charge of statistical analysis and all authors reviewed and contributed to the manuscript. All authors have read, approved the paper and meet the criteria for authorship as established by the International Committee of Medical Journals Editors.
Funding This work was supported by a grant from the French Ministry of Health (APJ 2019, registration number: N07).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.