Article Text
Abstract
Introduction Children with cerebral palsy (CP) classified as gross motor function classification system (GMFCS) levels III–IV demonstrate impaired sitting and reaching control abilities that hamper their overall functional performance. Yet, efficacious interventions for improving sitting-related activities are scarce. We recently designed a motor learning-based intervention delivered with a robotic Trunk-Support-Trainer (TruST-intervention), in which we apply force field technology to individualise sitting balance support. We propose a randomised controlled trial to test the efficacy of the motor intervention delivered with robotic TruST compared with a static trunk support system.
Methods and analysis We will recruit 82 participants with CP, GMFCS III–IV, and aged 6–17 years. Randomisation using concealed allocation to either the TruST-support or static trunk-support intervention will be conducted using opaque-sealed envelopes prepared by someone unrelated to the study. We will apply an intention-to-treat protocol. The interventions will consist of 2 hours/sessions, 3/week, for 4 weeks. Participants will start both interventions with pelvic strapping. In the TruST-intervention, postural task progression will be implemented by a progressive increase of the force field boundaries and then by removing the pelvic straps. In the static trunk support-intervention, we will progressively lower the trunk support and remove pelvic strapping. Outcomes will be assessed at baseline, training midpoint, 1-week postintervention, and 3-month follow-up. Primary outcomes will include the modified functional reach test, a kinematic evaluation of sitting workspace, and the Box and Block test. Secondary outcomes will include The Segmental Assessment of Trunk Control test, Seated Postural & Reaching Control test, Gross Motor Function Measure-Item Set, Canadian Occupational Performance Outcome, The Participation and Environment Measure and Youth, and postural and reaching kinematics.
Ethics and dissemination The study was approved by the Columbia University Institutional Review Board (AAAS7804). This study is funded by the National Institutes of Health (1R01HD101903-01) and is registered at clinicaltrials.gov.
Trial registration number NCT04897347; clinicaltrials.gov.
- REHABILITATION MEDICINE
- Paediatric neurology
- Motor neurone disease
- Clinical trials
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This randomised controlled trial investigates an understudied subpopulation of individuals with cerebral palsy (CP).
The methodology details our novel seated postural and reaching control intervention for children with CP.
The study maximises the motoric benefits for both the experimental and control groups.
The methodology will elucidate the effect of active motor training while providing tailored postural trunk support.
The participation of children with CP and severe intellectual deficits will be limited.
Introduction
Cerebral palsy (CP) is the most common life-long childhood physical disability with 2.0–3.5 per 1000 births, and a lifetime cost per person of $921 000 in the USA.1 2 Approximately, 29% of these children have moderate-to-severe bilateral CP (BCP)—gross motor function classification system (GMFCS) levels III–V.3–5 Abnormal posture and motor deficits are some of the most disabling impairments.3 5 6 Yet, efficacious therapies targeting sitting postural control that result in long-lasting functional benefits are scarce.7 This is particularly problematic for children with BCP, GMFCS III–IV, who require sitting abilities for wheeled mobility, activities of daily living (ADLs), an active physical life and community participation.8–12 Sitting control deficits are commonly resolved by assistive systems and by modifying contextual factors (ie, power wheelchairs, head and lateral trunk supports, seating adaptations and personal assistance).8 13 This assistive approach facilitates participation; however, these children may not be performing at their maximal independent motor potential. Thus, promoting postural and reaching abilities during independent sitting are essential to enhance the functional life of these children. In this vein, what is the best evidence-based therapeutic strategy to improve seated functions in children with BCP?
Children with GMFCS III–IV show trunk control deficits at the middle and lower regions of the thorax as well as reaching impairments—as determined by the Segmental Assessment of Trunk Control (SATCo) and Seated Postural & Reaching Control (SP&R-co) tests.14 15 Consequently, changing an external support from mid-ribs to pelvis significantly decreases postural and reaching control in sitting.16 This suggests the potential application of external support at specific trunk levels to deliver seated postural interventions.17 18 A recent randomised controlled trial (RCT) in CP, GMFCS III–V, compared conventional therapy with a home-based activity training delivered with external support at the impaired trunk segment. The intervention resulted in significant short-term postural improvements (ie, sway) but not in long-term motor benefits.19 The absence of long-term effects may be because the intervention was not structured around motor learning and control principles, which are essential for inducing neural plasticity and lasting functional outcomes.20–24
In the present study, we have developed a robotic Trunk-Support-Trainer (TruST) to evaluate seated balance and implement a motor learning-based postural intervention (TruST-intervention).25 26 TruST is a motorised-cable-driven belt that applies force field technology. A key factor is that the force field matches the participants’ sitting stability limits to supplements their motor efforts when the trunk is beyond such postural limits. Thus, force fields are tailored to the postural ability of the participants as their postural control improves across intervention sessions (ie, postural task progression). Moreover, TruST displays real-time feedback about the trunk’s location with respect to the participant’s stability boundaries, allowing the clinician to target postural strategies within, at, or beyond sitting control boundaries. The current RCT investigates the efficacy of TruST-intervention compared with the same motor intervention implemented with a static trunk support system in children with BCP, GMFCS III–IV.
Aims and hypotheses
Overall aim
We will test whether a motor learning-and-control-based intervention can improve seated postural and reaching abilities in children with BCP, GMFCS III–IV.
Primary hypotheses
We expect improvements with TruST and the static trunk support system. However, we hypothesise greater postural improvements with TruST-intervention, as shown by larger improvements in a customised postural-star sitting test (PSST) and the modified functional reach test (mFRT). Regarding upper extremity control, we expect improvements with both interventions, as determined by the Box and Block (B&B) test and video-coding analysis.
Secondary hypothesis
We expect improvements in both intervention groups. However, we expect greater improvements with TruST-intervention in segmental trunk control (SATCo), postural sitting and reaching control (SP&R-co), gross motor function (Gross Motor Function Measure-Item Set, GMFM-IS), child-centred and family-centred functional and participation outcomes (Canadian Occupational Performance Outcome, COPM, and The Participation and Environment Measure and Youth, PEM-CY), and in postural and reaching kinematics.
Methods
Study design
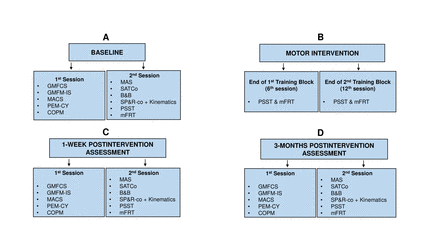
This is an explanatory parallel RCT conducted at Columbia University (New York) in 82 children with BCP, GMFCS III–IV, aged 6–17 years. The study timeline is from February 2022 to December 2026. After baselines, we will test improvements at mid-point of the intervention (sixth session), 1 week postintervention, and 3 months follow-up. The Consolidated Standards of Reporting Trials (CONSORT) will be followed.27 28 A patient or family advisory board did not participate during the planning of our RCT study.
Recruitment
Participants will have a confirmed medical diagnosis of BCP. They will be recruited by advertising our study on our website and others, social media platforms, clinicaltrials.gov, and through various local clinics and school districts in New York. Testing and training sessions will be adjusted to the family’s schedule before starting the study. During an initial prescreening, a phone survey will be scheduled to interview families, caregivers or legal guardians by KC or VS. We will obtain information beforehand on participants’ eligibility criteria and discuss our study design, research goals, potential risks, and reciprocal commitment with participants and families. We expect that our recruitment strategies and participants eligibility will maximise retention and intervention benefits.
The participants will meet the following inclusion criteria to participate in our study: (1) age 6–17 years; (2) medical diagnosis of BCP (diplegia, triplegia or quadriplegia); (3) GMFCS levels III or IV; (4) ability to sit 5s with manual support provided to any trunk region at or between mid-ribs and pelvis (SATCo=3–7), and (5) cognitive capacity to follow basic verbal instructions (eg, ‘do not put your hands on your lap’, ‘keep your hands up in the air’ or ‘follow and reach or touch the toy’). Exclusion criteria include: (1) absent head control (SATCo=1); (2) current medical illness unrelated to CP at the time of the study; (3) severe dyskinesia that impedes the child to sit and/or perform postural and/or reaching movements; (4) history of recurrent seizures (daily) or refractory epilepsy; (5) severe structural deformities of the spine (scoliosis >40◦ and/or kyphosis >45◦); (6) orthopaedic surgery of the spine and/or upper and/or lower extremities in the last 6 months before the study onset; (7) severe spasticity of biceps/triceps in both upper extremities that prevent reaching movements (Modified Ashworth Scale (MAS)=4); (8) chemodenervation or neurolysis (eg, botulinum toxin or phenol/ethyl alcohol injections) in the upper or lower extremity muscles 3 months before the study or planned during the duration of the study and (9) major surgeries in the previous 6 months (only if medically contraindicated).
Randomisation and participant allocation
A researcher external and blinded to our study will create computer-generated lists of random numbers assigned to seven blocks of 10 participants and to one block of 12 participants (n=82). To prevent selection bias, the allocation sequence will be concealed from the research team. After randomisation to either the TruST-intervention or static trunk support-intervention group, the independent researcher will communicate to the research team the assigned group by opaque, sealed envelopes. Carbon paper inside the envelope will be used to transfer the information onto an allocation card that will be kept with the participant’s records. The envelopes will be opened after the consent of the enrolled participant and the completion of baseline assessments.
Blinding
All assessments will be videotaped, performed and scored by clinical evaluators with expertise in CP. The evaluators will be blinded to group allocation and testing sessions. Blinding of families and children to the intervention will not be possible due to equipment characteristics—that is, robotic-TruST versus static trunk support system.
Study locations
Both intervention arms will be delivered at Columbia University (New York). The TruST-intervention will take place at the Robotics and Rehabilitation Laboratory; whereas, the static trunk support-intervention will be carried at the Center for Cerebral Palsy (Teachers College). The same research personnel will collect data and deliver the motor interventions. However, clinical evaluators will be different personnel and blind to participant allocation.
Study interventions
Participants will concurrently follow their regular therapeutic care during the study, which will be documented. The TruST-intervention and static trunk support-interventions are detailed in table 1, following the Template for Intervention Description and Replication (TIDieR) Checklist.29 30 The same motor learning and control principles, and activities will be applied to both interventions.25
TiDiER checklist for comparison between TruST intervention and static trunk support interventions
Common intervention procedures: TruST- & static trunk support-interventions
Dosage
The dosage for both interventions will be identical, 2 hours/session, 3 x/week, for 4 weeks (12 training sessions). In our previous study,25 we found the proposed intervention schedule and dosage to be effective in promoting short-term and long-term improvements in seated postural and reaching abilities and gross motor functions.
Therapeutic approach
In both intervention groups, all motor activities will be trained along eight star-radiated directions spaced at 45° and with the centre at the participant’s pelvis. The goal of this postural intervention scheme is to cover the 360° peripersonal space around the seated participant while being trained at different reaching distances (figure 1A).
(A) depicts the star-shaped scheme applied during the motor intervention with TruST and rigid trunk support systems. The postural star-sitting test follows the same scheme used to compute sitting workspace area (cm2). (B) shows a model of TruST with a child. The main components are numbered: motors (1), pulleys and cable tension sensors (2), cables (3), mechanical lifting platform (4), bench with pelvic strapping (5) and ball used during the postural star-sitting test (6). The arrow depicts the active trunk excursion. (C) depicts the static trunk support system and the main components: principal rigid column (1), U-shaped trunk support that slides along the vertical column (2), trunk support adjustments in the frontal and sagittal planes (3), base of the frame with wheels that can be locked (4). Note that the frontal belt and bench are not shown in this model. TruST, Trunk-Support-Trainer.
Activities will be practised under moderate–high intensity but never beyond extreme fatigue, as reported by the child or by the presence of clinical signs such as muscle trembling. Any potential pain or discomfort will be monitored with the Wong-Baker Faces pain scale during and after the intervention in each study session.31
Parameterisation of the motor intervention
The motor intervention parameters have been investigated in previous studies (table 2).25 32 A subset of modified motor parameters defined by Fleishman (1972) will be used to modulate postural and reaching control strategies during the motor intervention.33 Motor learning-based interventions depend on participants’ own preference, motivation and cognitive-motor abilities. Thus, these parameters will be adjusted across participants and intervention sessions.20 22 34
Activities and motor learning and control parameters
Mode of intervention delivery and setting
One-to-one interventions will be delivered in a lab setting by a physical or occupational therapist. All research personnel will be trained and supervised. A pediatric physical therapist and researcher (VS) will provide direct supervision every two intervention sessions. Also, a bioengineer (XA) will operate TruST while another researcher/clinician collects kinematic data or delivers the motor intervention.
Postural-task progression procedures
TruST-intervention: postural assistive-force fields
The TruST-belt will be placed on the lower ribs (T9-12) to provide assist-as-needed forces. The PSST will be used to match the assistive force tunnel to the participant’s sitting control boundaries and measure sitting workspace (cm2).25 35 This test is based on the Star Excursion Balance Test; in which the person displaces the foot along eight directions, following the shape of a star during one leg stance.36 Similarly, the PSST is a game-oriented test, in which the seated participant performs maximal trunk excursions. A large ball is presented nearby the participant’s face to guide the eight trunk movements that radiate in a star-like fashion. After each maximum trunk displacement, the participant needs to recover the original upright sitting posture without using the hands for support.
During the TruST-intervention, the assistive-force field intensity equals 10% of the child’s body weight (figure 1B). These forces assist sitting balance towards the predefined stability boundaries and not to the centre of the star-shaped region. Moreover, assistive forces are only provided when the trunk is beyond the boundaries to supplement the participant’s motor efforts. This configuration promotes continuous active sitting control without hand support to practice goal-oriented tasks. As the participant expands the sitting control area across intervention sessions, the assistive-force field boundaries are increased and matched to the new stability boundaries (ie, postural-task progression).
Another critical parameter to the achievement of independent sitting will be the removal of pelvic strapping (ie, unsupported sitting). We will follow one of two criteria to remove the straps. The child shows a pretraining sitting workspace area above two SEs of the mean from the previous two, or more, pretraining sessions; or pelvic strapping is removed after the sixth session. Our previous study suggests that participants will likely acquire unsupported sitting (unstrapped) by the sixth intervention session.
Static trunk support-intervention: segment-by-segment approach
The static trunk support system (figure 1C) design follows engineering principles, kinematic and electromyographic data in sitting and reaching control that apply to healthy adults, developing infants, and children with CP.16 18 19 37–41 As determined by the SATCo test, we will follow a top-down segment-by-segment approach to evaluate trunk control in sitting at the beginning of each intervention session. We will define the most-impaired trunk segment, place the support, and then deliver the motor intervention. The constraint of caudal trunk segments to the one being trained might help to reduce the overload of sensorimotor information to process and to control the body dynamics during seated motor activities.37 41 However, legs and feet will not be supported.
For postural task progression, when there is an improvement in the SATCo—that is, improved sitting balance at a lower trunk segment—the support is lowered one level. The trunk support system will offer a firm support for a systematic, objective and reliable SATCo evaluation across participants and sessions.
Discontinuation criteria for motor interventions
We will discontinue the TruST-intervention if postural detriments are observed—that is, workspace area decreases during 3 consecutive days below 2 SE of the averaged preintervention sessions prior to the session when the detriment onset is detected. Static trunk control-intervention will be discontinued if the SATCo score decreases one level, or more, for 3 consecutive days. Any intervention will stop if the participants report excessive pain (visual analogue scale≥7).
Motor-task progression procedure
In the TruST-intervention, motor training will be progressed as follows:
Within sitting boundaries (inactive TruST-force field): The participant performs 30–50 simple reaches (ie, pointing) with the less-impaired and more-impaired upper extremities. The target is placed at maximum active reaching distance without eliciting additional trunk movements on the right and left sides of the body, following eight star-like directions—as we follow in the postural star-sitting test. If 60% of attempts are successful in a minimum of five out of the eight directions (clockwise or counterclockwise), the participant progresses to stage 2.
Beyond sitting control boundaries (active TruST-force field): the target is placed beyond stability boundaries (~120% active reaching distance) along the eight star-like directions to elicit trunk movements. In this stage, the participant relies on assistive-force fields to complete the motor activity and return to sitting posture without using the hands to recover sitting stability. As in stage 1, the participant can progress to stage 3 when 60% of attempts are successful at least in five out of the eight directions (clockwise or counterclockwise).
Beyond sitting control boundaries under challenging motor conditions: the training procedure is like stage 2. However, in stage 3, the clinician modulates specific motor control parameters (see table 2 above), adds practice variability—movement distance and directionality—and introduce diverse goal-oriented activities (ie, contextual interference) to address maximum motor complexity.
In the static trunk support group, we will follow the same motor skill training and stages. However, in stages 2 and 3, the participants will rely on a static trunk support to perform the postural and reaching activities without the additional use of the hands for support.
Adverse events and safety
As per our IRB protocol, major risks or serious long-term harm are not expected. Thus, pre-established compensation has not been determined. Major falls from the bench will be prevented with a slacked harness—to avoid weight support during the intervention. Minor equipment—or intervention-related injuries that do not require medical attention are muscle fatigue, minor dermic abrasions, and localised erythema or petechiae under the belt or trunk support. If adverse events such as muscle or articular pain, excessive physical or cognitive fatigue, musculotendinous strains or ligament sprains occur, these will be documented in our study protocols (see the Fidelity section) and IRB.
Fidelity
Supervisory team: researchers’ attributes, scientific documentation and personnel training
We will have a Manual of Procedures (MOP) in place that covers each treatment arm. The MOP will describe the study design, personnel roles, experimental procedures, interventions, data analyses and safety measures, and how to handle blinded and private data. We will register in the study MOP adverse events and protocol or procedure modifications.
All research personnel (including volunteers) in direct contact with participants will receive training in ethical, safety, experimental, and intervention protocols to achieve optimal ethical and professional attributes to perform the study. This training will include IRB-related coursework (eg, ‘Good Clinical Practice’), basic first aid, and cardiopulmonary resuscitation (CPR) training. It will also include communication skills to interact with participants and families, information on RCT designs—ensuring internal and external validity of the study—and a 2-hour in-person training seminar to learn about postural-related and reaching-related deficits in CP, our motor intervention design and how to operate the TruST and static trunk support systems.
Data monitoring during the study
Attendance will be used to measure participation and monitor potential dropouts, including if the reason is internal or external to our study. Video footage of training sessions will be video coded to determine training effectiveness (ie, time-on-task), type and frequency of motor activities practised, toys or objects used, and motor capacity (eg, success to achieve the goal, time to achieve the task, and number of task repetitions). An external researcher with expertise in video-coding analyses, who is independent to our study team, will analyse masked video data with Datavyu software (https://datavyu.org/).
A data monitoring committee has not been established. In weekly meetings, we will monitor whether all study protocols are implemented as planned. Aside from an external statistical analysis, interim statistical analyses will be carried out to monitor the progression of the two study arms. If 50% of the projected sample size does not improve in either intervention, we will inform the funding agency and discontinue our RCT.
Participant’s data
Using the ICF framework, we will collect data within the body structure and function, activity and participation domains.13 Figure 2 depicts the study outline and data collection.
Diagram depicting the study timeline and type of data gathered in each study phase. B&B, Box and Block; COPM, Canadian Occupational Performance Outcome; GMFCS, gross motor function classification system; GMFM-IS, Gross Motor Function Measure-Item Set; MACS, Manual Ability Classification System; MAS, Modified Ashworth Scale; mFRT, modified functional reach test; PEM-CY, Participation and Environment Measure and Youth; PSST, postural-star sitting test; SATCo, Segmental Assessment of Trunk Control; TruST, Trunk-Support-Trainer; SP&R-co, Seated Postural & Reaching Control.
Medical, demographic and concurrent therapy data
Demographic questionnaires used by the National Institutes of Health will be used to gather data on sex, age, race and ethnicity. This data will be used to ensure racial and ethnic diversity. Medical information such as CP diagnosis and subtype, brain injury and other comorbidities will be obtained from medical records. We will record the current medical and therapeutic regimens—type, schedule and intensity—of participants for further interpretation of our study outcomes. Any communication that involves personal or medical information will follow the Health Insurance Portability and Accountability Act of 1996 (HIPAA).42
Screening and descriptive measures
Gross motor function classification system
The GMFCS comprises five levels of severity. It categorises functional abilities such as sitting, walking, running or jumping while considering the need for assistive equipment (postural support, wheeled mobility, or walkers).43
Manual Ability Classification System
The Manual Ability Classification System categorises how children manipulate objects during ADL depending on their functional independence.44
Spasticity will be measured with the MAS
The MAS can be used to assess spasticity in CP.45 46 It scores the increase in muscle resistance through passive limb movements. The score ranges from 0 (no increase in muscle tone) to 4 (limb rigid in flexion or extension). We will be cautious interpreting spasticity as MAS scores depend on joint and muscle features, and examiners’ experience.46
Primary outcomes
Modified functional reach test
The mFRT measures proactive postural control during maximum reaching distance. It is a reliable tool in CP (r=0.42 to 0.77) and discriminates GMFCS levels (GMFCS III=10.8 cm ± SD: 3.8).47 48 Test responsiveness is unknown in CP, however.
Postural-star sitting test
It will be performed before and after interventions to monitor sitting control progression in both TruST-intervention and static trunk control-intervention groups. The investigators have several motivations that rationalise this customised measurement. It (1) is age appropriate, (2) is goal oriented, (3) directly measures sitting based on trunk control improvements, (4) is responsive to capture sitting workspace area chnages and (5) offers data with a straightforward functional interpretation.
Box and Block
It examines manual dexterity. The child moves a maximum number of blocks (block size=2.5 cm2), one at a time, between the compartments of a partitioned box in 60s.49 In BCP, B&B shows a strong association (r≥0.7) with self-care, mobility and social function.50 B&B is responsive to motor interventions that include more-affected and less-affected hands with a minimal clinically important difference (MCID) of 1.9 and 3.0 blocks, respectively.51 52 Arm displacement and grasping will be analysed with Datavyu.53 An instruction manual has been created to standardise video-coding procedures and define the reaching variables. Grasping is defined as the moment the hand contacts the block to the time the block is lifted from the surface. Arm displacement is defined from end of grasping to block release. Reaching performance is the summation of grasping and arm displacement. Two, or more, coders will determine video-coding reliability (r≥0.7).
Secondary outcomes
Gross Motor Function Measure-Item Set
The GMFM-IS determines the gross motor function of children with CP—A: lying and rolling, B: sitting, C: crawling, D: standing and E: walking, running and jumping. It is an abbreviated and validated version of the GMFM-66. It includes an algorithm with three critical items to decide which one of four item sets is most appropriate for the child to assess motor function and obtain a GMFM-66 score.54 GMFM shows strong inter-rater reliability (к=0.75) for 2–12 years and strong inverse correlation with GMFCS (r=−0.91).55 56 Moreover, it is responsive to change with an MCID of 0.8–1.6 (medium effect size) and 1.3–2.6 (large effect size).57
Canadian Occupational Performance Outcome
The COPM will be used to measure parent-centred and child-centred functional goals and preferences specific to seated posture and reaching impediments that restrict participation.58 COPM has high inter-rater agreement in prioritising problems (80%) and it can detect clinical important differences across time (ie. a MCID above two-point change).59–61
Participation and Environment Measure and Youth
The PEM-CY measures participation—12 home items, 17 school items and 16 community items—including environmental factors (reliability: home=0.71, school=0.76 and community=0.69).62 63 A study on one environmental-based intervention showed that PEM-CY can capture improvements in children with physical disabilities. We will explore whether PEM-CY can capture postintervention changes in our study.64
Seated Postural & Reaching Control test
The theoretical play-oriented framework and metrics of the SP&R-co test have been validated in children with CP who have moderate-to-severe motor conditions. It shows good–excellent inter-rater and intrarater reliability (Intraclass Correlation Coefficients=0.68–0.86, and 0.64–0.95, respectively). As the SATCo, the SP&R-co follows a segment-by-segment trunk approach to assess quantitatively sitting control across static, active, proactive (via bimanual and unimanual reaches) and reactive dimensions. Responsiveness has not yet been addressed, but the SE measurements for each seated postural dimension of the SP&R-co test are available.14
Postural and reaching kinematics
We will follow the seated postural framework validated in the SP&R-co to capture motor improvements in the next tasks:
Static seated task: Postural orientation and balance in sitting during 20 s.
Active seated task: Simultaneous control of the trunk and head rotations when the child visually follows an object 90° to the right and left (ie, chin over shoulder).
Proactive seated task: Seated anticipatory and compensatory postural control during direction-specific reaches performed straight, and 45° to the right and left.
Segmental Assessment of Trunk Control
The SATCo is validated in children with CP and shows excellent inter-rater and intrarater reliability (ICCs >0.84 and 0.98, respectively). The evaluator offers support at various trunk segments (shoulders, axillae, inferior angle of scapulae, on lower ribs, below lower ribs and pelvis) to measure trunk control across three dimensions: static (during 5 s), proactive (visually following an object to the right and left) and reactive (postural responses to nudges). We will consider a score from 1 (no head control) to 8 (full trunk control).15 Test responsiveness has not been established, but studies show potential to identify trunk balance improvements in each of the tested trunk segments.18 41
Data management and data collections
After the subject eligibility is confirmed, we will assign a code to each participant only accessed by the principal investigators (SKA and AMG), coinvestigator (VS) and research coordinator (KC). All data collections will be digitised and saved in encrypted endpoint hard drives. Paper forms will be collected as safe copies in a private locked cabinet in the PI’s office.
To keep young children informed and engaged during the study, each one will receive a personalised fun ‘Research Passport’ that lists each study stage and explains the purpose of each visit. On completion of each study phase and procedure, the child will earn a stamp on each page. Additionally, we will offer families the possibility of receiving a brief clinical informative report with the functional status of the child after the study by VS—who is a licensed board-certified paediatric physical therapist in New York.
We will divide our three main data collection events (baseline, 1-week postintervention and 3-month follow-up) into two subsessions to reduce the burden and physical fatigue that the evaluations may cause (figure 2). We will empower participants with the ability to stop any study session and request breaks verbally or with a laminated red stop sign.
Data analysis
Sample size estimation
We used preliminary data from our previous study and literature to estimate sample and effect sizes.16 25 For this purpose, G-Power (V.3.1.9.4., Dusseldorf University) and SPSS (V.25, IBM) were used. Our primary outcome was upper body balance during seated reaching (Pilot average=30° ± SD=22°, partial η2=0.10, n=11). With a mixed analysis of variance (ANOVA), we estimated 68 subjects to achieve a power=0.8, considering a two-tailed α rate=1% (p<0.005). We will recruit an additional 20% of participants (82 participants in total) to account for potential group heterogeneity and dropouts.
Statistical procedures
An alpha rate=0.01 will be used for statistical analyses. The effect of the interventions on primary and secondary outcomes will be analysed with a two-factor mixed ANOVA, including groups as the between-subject factor (TruST and static trunk support groups), and testing sessions as the repeated measures factor (baseline, mid-point training, 1-week postintervention and 3 month follow-up). The group X testing session interaction will be used to test the hypothesis that TruST-intervention is superior to static trunk support intervention. If the ANOVA model is significant, we will perform post hoc comparisons with Holm-Bonferroni procedure to control family-wise error.
Statistical handling of non-normally distributed and missing data
In the event that participants miss sessions for unpredicted reasons (eg, illness) or drop the study, we will apply a generalised estimating equations (GEE) as an alternative statistical plan. In this way, we will account for missing data and follow an intent-to-treat principle. The GEE will analyse events-in-trials following a repeated-measures procedure with subjects as clusters, test session as the within-subject variable and intervention groups as the between-subject variable. A linear model will be selected, and the covariance structure will be specified as correlation matrix based on the quasi-likelihood under independence criterion goodness of fit coefficient.65
Ethics, resource sharing plan and dissemination
The present RCT has been registered on clinicalgov.org. The study protocol, recruitment materials, and assent and consent forms have been approved by the Columbia University Institutional Review Board (IRB AAAS7804). Study information, assent and informed consent forms will be signed by all participants and caregivers prior to requesting medical records and starting the study. Participants will be verbally reminded they can withdraw consent at any time without penalty. All deidentified data will be stored for 3 years after the completion of the study in a password-protected computer. We will store deidentified data in an online HIPAA-compliant database (REDCap). The study protocols follow standardised procedures in RCT such as CONSORT and TIDieR to facilitate appropriate scientific, ethical and safety assessments and to increase the likelihood of research success.27 29 30
We will make available the study data via the Data and Specimen Hub—a data sharing platform of the Eunice Kennedy Shriver National Institute of Child Health and Development. Findings will be disseminated through peer-reviewed publication and national and international conferences. Participants and families will be informed on the study progress via newsletters and meetings.
Discussion
We are expanding on our previous small feasibility study in which we did not include a control group (ie, static trunk support-intervention).25 We expect our motor learning-based postural interventions to induce postural and reaching improvements in both study groups. Nonetheless, we expect that postural-task progression tailored to the participant’s postural stability via TruST-force fields will have a synergistic effect during motor training that may lead to greater improvements. As shown in our previous studies, we will apply motor-task progression to challenge the child via specific motor parameters during age-appropriate and goal-oriented activities that maximise engagement. Tailored postural support that is progressively lowered allows participants to experience a full motor repertoire based on self-initiated movements and trial-and-error practice. We do not expect safety concerns during the motor interventions but physical fatigue is highly plausible due to motor-task and postural-task progression. If our hypothesis is supported, a critical point will be knowledge translation of TruST-intervention. Valid static trunk support systems are accessible in most rehab settings and special education schools. Regarding TruST, the team will investigate its development into a versatile and affordable equipment with an user-friendly interface for future clinical applications. In future studies, we will address whether a distributed motor practice, more similar to regular therapy schedules (30–60 min vs 120 min), could be equally effective. Finally, if participants acquire the ability to sit unsupported, further studies will be necessary to objectively identify how to modify the child’s context (physical barriers) to fully transfer and retain the seated functional gains across ADLs.
Ethics statements
Patient consent for publication
Acknowledgments
We thank all participants and families who collaborated in our pilot studies and offered feedback to design the present RCT. We thank Dr. Jaya Rachwani for her valuable insights and revisions on video-coding procedures during the B&B test.
References
Footnotes
Contributors As per ICMJE criteria, all authors have contributed to the manuscript. Specific contributions: SKA and AMG are the principal investigators. VS is the co-investigator. SKA, AMG, JPD and VS have designed the RCT and standardised study procedures and training personnel documentation. VS trains research personnel in motor intervention protocols. SKA, AMG and VS supervise data collections. KC is the research coordinator. XA is the PhD candidate and bioengineer during data collections. SKA, AMG, JPD, VS and XA will process, analyse and interpret the data. SKA, AMG, VS, XA and KC will collaborate in the final scientific write-up of the research work.
Funding This work is supported by the National Institutes of Health (1R01HD101903-01). NIH grants the funds and will annually review research progress. NIH will make our research work publicly available.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.