Article Text
Abstract
Objectives To evaluate the efficacy and safety of anti-vascular endothelial growth factor (anti-VEGF) therapy for myopia choroidal neovascularisation (CNV), and to compare the efficacy of two different anti-VEGF retreatment criteria.
Data sources PubMed, EMBASE, the Cochrane Library and ClinicalTrials.gov were searched from inception to 31 July 2022.
Study selection Randomised controlled trials (RCTs) comparing anti-VEGF with sham, photodynamic therapy (PDT) or PDT combination therapy in patients with myopia CNV were reviewed and selected. RCTs comparing visual acuity (VA) stabilisation or disease activity as anti-VEGF retreatment criteria were also included in the study.
Data extraction and synthesis Two reviewers independently conducted data extraction and quality assessment. We used a random-effects model for all analyses. Primary outcomes included best-corrected visual acuity (BCVA) and central foveal thickness. Secondary outcomes included number of patients who gained more than three lines in BCVA, number of anti-VEGF injections and ocular adverse event (AE).
Results Seven RCTs involving 1007 patients were included. Compared with sham and PDT therapy, anti-VEGF therapy achieved better BCVA gains of −0.28 logMAR (95% CI −0.36 to −0.20, p<0.00001) and −0.14 logMAR (95% CI −0.17 to −0.10, p<0.00001), respectively. Both ranibizumab and bevacizumab improved patients’ vision better than PDT therapy and no definitive increased risk of ocular AE was observed. Analysis of two small RCTs showed that PDT combination therapy had similar visual improvement and needed fewer anti-VEGF injections compared with anti-VEGF monotherapy (weighted mean difference (WMD)=1.30; 95% CI 1.24 to 1.37, p<0.00001). Anti-VEGF retreatment guided by disease activity criteria resulted in comparable visual improvement and reduced anti-VEGF injections compared with retreatment guided by VA stabilisation (WMD=0.83; 95% CI 0.42 to 1.25, p<0.0001).
Conclusions Anti-VEGF therapy is effective and well-tolerated for myopia CNV patients. Anti-VEGF retreatment guided by disease activity criteria can achieve comparable efficacy and potentially reduce anti-VEGF injections.
PROSPERO registration number CRD42021292806.
- ophthalmology
- clinical pharmacology
- medical retina
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This meta-analysis included all available data from the most recent randomised controlled trials (RCTs) and comprehensively compared anti-vascular endothelial growth factor (anti-VEGF) with different treatment strategies for myopic choroidal neovascularisation.
Our review included multicentre RCTs comparing the efficacy and number of injections of disease activity and visual acuity stabilisation as anti-VEGF retreatment criteria to recommend superior anti-VEGF retreatment criteria.
The number of included RCTs was relatively small, and some RCTs had small sample sizes, requiring larger relevant studies.
The inconsistent follow-up time points may account for the heterogeneity of some parameters, which limits the generalisability of the study results.
Introduction
Pathological myopia is characterised by excessive elongation of the eyeball, leading to various degenerative changes in the retina and visual deterioration.1 Among the complications of pathological myopia, choroidal neovascularisation (CNV) and mechanical rupture of Bruch membrane are the most serious degenerative changes.2 Pathological myopia is the second cause of CNV after neovascular age-related macular degeneration, with approximately 5.2%–11.3% of pathological myopia patients developing to myopic CNV.3 4 Myopic CNV has a higher prevalence in Asian population, with most patients developing the disease at age 50 or younger, rather than in old age.5 Without treatment, the majority of myopic CNV patients will develop a poor visual outcome. A 10-year follow-up study showed that over 95% of myopic CNV patients had reduced visual acuity (VA) to 0.1 or worse at 5 and 10 years after onset.6
Before the use of anti-VEGF therapy in myopic CNV, treatment strategies mainly included laser photocoagulation, verteporfin photodynamic therapy (PDT) and submacular surgery.7–10 However, the clinical application of these approaches is limited by complications such as myopic CNV recurrence, scarring, atrophy and choroidal ischaemia.7 11 12 PDT has been the most widely used treatment for myopic CNV since the verteporfin in PDT (VIP) study showed that patients treated with PDT had better visual outcomes over 12 months compared with placebo.8 However, the 2-year follow-up of the VIP trial reported no statistically significant benefit from PDT treatment and a high recurrence rate of intraretinal fluid after treatment.9 Another study showed that 83% of PDT treated patients developed choroidal atrophy after 5 years.13 Since anti-vascular endothelial growth factor (anti-VEGF) therapy become available, PDT has fallen out of favour and only considered when anti-VEGF therapy is contraindicated.
VEGF, a proangiogenic cytokine that stimulates the development of CNV, is abnormally increased in the eyes of myopic CNV patients.14 Anti-VEGF binds to VEGF receptor to inactivate endogenous VEGF and inhibit the migration and proliferation of vascular endothelial cell, thereby inhibiting neovascularisation.15 The earliest report of intraocular injection of anti-VEGF drugs for myopic CNV was in 2006 and has been increasingly used in recent years.16 17 Although previous studies have shown that anti-VEGF therapy leads to better vision, comparative studies mainly consist of non-randomised controlled trials (non-RCTs) and a small number of RCTs, which limits the strength to support clinical application.18 19 Furthermore, despite clinical approval of anti-VEGF therapy for myopia CNV, the optimal retreatment criteria have not been unified.20
In recent years, new RCTs about anti-VEGF therapy for myopia CNV have been published and long-term data on efficacy and safety have been accumulated. Most importantly, two large RCTs have been completed to compare the therapeutic effects of different anti-VEGF retreatment criteria.21 22 Our aim was to update the latest clinical evidence and to explore preferred anti-VEGF retreatment criteria for myopic CNV.
Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline.23
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Data sources and search strategy
The databases of PubMed, EMBASE, the Cochrane Library and ClinicalTrials.gov were searched from inception to 31 July 2022. A range of MESH words and free terms regarding CNV, anti-VEGF, ranibizumab (Lucentis), bevacizumab (Avastin), aflibercept (Eylea), conbercept (Lumitin), RCT were used in all possible combinations to search for relevant articles. The search strategy is provided in online supplemental material 1. No language restriction was applied. We also manually searched the reference lists of included studies to identify other potentially eligible articles.
Supplemental material
Eligibility criteria
We included the following published studies if they met the criteria: (1) patients with active myopia CNV (with spherical equivalent ≥−6.0 dioptres and an axial length ≥25.0 mm); (2) studies were RCTs that directly compared intravitreal anti-VEGF drugs with sham or PDT or PDT combination therapy for the treatment of patients with myopia CNV; (3) RCTs comparing VA stabilisation or disease activity as anti-VEGF retreatment criteria were included, with VA stabilisation criteria was defined as no change in best-corrected visual acuity (BCVA) as compared with the two preceding monthly visits and disease activity criteria was defined as vision impairment attributable to intraretinal or subretinal fluid or active leakage secondary to myopia CNV; (4) studies reported one or more of interest outcomes. Exclusion criteria were employed as follows: (1) patients were previously treated with several drugs; (2) comparative studies between different anti-VEGF drugs, non-comparative studies, animal studies or case reports; (3) unfinished studies or unavailable data.
Data extraction and quality assessment
Titles and abstracts were scanned independently by two reviewers using the selection criteria described above. Disagreements were discussed and if necessary, resolved by a third reviewer. Data were extracted in a prespecified data extraction form. The following data were extracted from the included articles: general data (title, first author, study design, inclusion and exclusion criteria), basic characteristics (age, sex, sample size), intervention groups, follow-up time, primary outcomes (BCVA and central foveal thickness (CFT)) and secondary outcomes (number of patients who gained more than three lines in BCVA, number of anti-VEGF injections and number of serious or non-serious ocular adverse events (AEs)). The quality of the RCTs was assessed using the Cochrane risk of bias tool.24
Data synthesis and statistical analysis
The meta-analysis was conducted using Review Manager V.5.3 supplied by Cochrane Collaboration (Oxford, UK). The weighted mean difference (WMDs) with 95% CIs were measured for continuous data, while the risk ratios (RRs) with 95% CIs were measured for dichotomous data. Visual outcomes were measured using the Early Treatment Retinopathy study chart and the data were converted to logarithmic VA (logMAR) for analyses.25 26 Heterogeneity between studies was assessed using the I² test. I²>50% was defined as the presence of substantial heterogeneity.27 Due to the possibility of heterogeneity being present between studies, a more conservative version of the random-effects model was applied. A value of p<0.05 was chosen as the significance level for outcome measures.
Results
Literature search
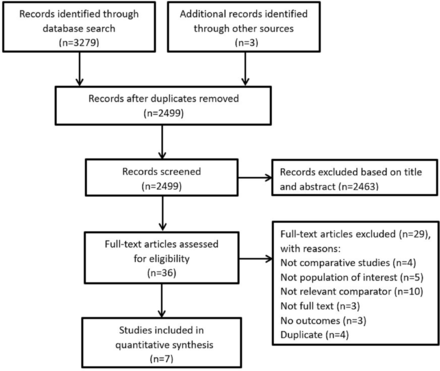
A total of 3376 relevant articles were initially identified. After removing 841 duplicates, we screened the remaining 2535 articles and excluded 2497 articles based on the titles and abstracts. The remaining 38 articles were retrieved for full-text review, and seven eligible RCTs21 22 28–32 were included in the meta-analysis (figure 1). Among the seven RCTs included, one RCT compared anti-VEGF with sham treatment, four RCTs compared anti-VEGF with PDT and two RCTs compared anti-VEGF monotherapy with PDT combination therapy. Besides, two RCTs compared different anti-VEGF retreatment criteria guided by VA stabilisation criteria or disease activity criteria, respectively.
Flow diagram of study selection process that was conducted in PubMed, EMBASE, the Cochrane Library and ClinicalTrials.gov.
Study characteristics
The basic characteristics of seven RCTs included are shown in table 1. The study included a total of 1007 participants. The followed up duration was 12–24 months. The mean age ranged from 44.6 to 62.4 years, with 52.5%–76.5% of female. The anti-VEGF treatments used in the included studies were intravitreal bevacizumab (1.25 mg), ranibizumab (0.5 mg) and aflibercept (2.0 mg). The PDT monotherapy received standard fluence PDT (50 J/cm2), and the PDT combination therapy received reduced fluence PDT (25 J/cm2) in combination with intravitreal anti-VEGF.
Characteristics of the included seven studies
For different anti-VEGF retreatment criteria, patient retreatment guided by VA stabilisation criteria received anti-VEGF on day 1 and month 1, followed by monthly injections when there was a loss of BCVA. Patient retreatment guided by disease activity criteria received anti-VEGF on day 1, followed by monthly injections when disease activity was observed.
Risk of bias assessment
Risk of bias assessment for included RCTs is shown in online supplemental material 2. Two RCTs21 22 were considered to be at low risk of bias for all domains. Most unclear risk of bias was assigned in domains of selection bias or detection bias.28 29 31 Two RCTs30 31 were considered to be at high risk of bias for performance bias and attrition bias, respectively.
Supplemental material
Anti-VEGF therapy versus sham
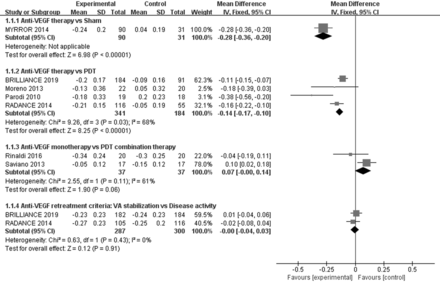
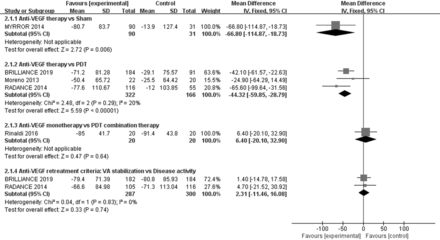
MYRROR study28 compared aflibercept with sham treatment, and results were presented at the end of 6 months because sham group could receive aflibercept when needed. The results showed that compared with the sham group, patients in anti-VEGF treatment achieved significant better BCVA (WMD=−0.28 logMAR; 95% CI −0.36 to −0.20, p<0.00001; figure 2) and CFT reduction (WMD=−66.80 µm; 95% CI −114.87 to −18.73, p=0.006; figure 3). The number of patients who gained more than three lines in BCVA was significantly higher in the anti-VEGF treatment than in the sham treatment group (RR=4.02, 95% CI 1.33 to 12.15, p=0.01; online supplemental figure). BCVA was significantly improved in patients treated with anti-VEGF compared with the sham group (−0.24±0.20 logMAR vs 0.04±0.19 logMAR), and a greater proportion of patients achieved more than three lines in BCVA (38.89% vs 9.68%). In addition, anti-VEGF-treated patients had a substantially larger mean decrease in CFT than sham patients (−80.7±83.7 µm vs −13.9±127.4 µm).
Supplemental material
Forest plot of studies examining the mean change in best-corrected visual acuity (logMAR). anti-VEGF, anti-vascular endothelial growth factor; PDT, photodynamic therapy; VA, visual acuity.
Forest plot of studies examining the mean change in central foveal thickness. anti-VEGF, anti-vascular endothelial growth factor; PDT, photodynamic therapy; VA, visual acuity.
The incidence of serious (p=0.55; table 2) and non-serious ocular AEs (p=0.13; table 2) were similar in anti-VEGF and sham treatment groups. There were three serious ocular AEs (only one macular hole in study eye) in anti-VEGF group and no event occurred in sham treatment group. The most common non-serious ocular AEs in anti-VEGF treated patients were mild conjunctival haemorrhage, punctate keratitis, eye pain and dry eye, but did not lead to the interruption of treatment.
Meta-analysis results of the number of anti-VEGF injections, serious and non-serious ocular adverse events
Anti-VEGF therapy versus PDT
Four RCTs21 22 29 30 compared anti-VEGF with PDT treatment, with two studies comparing ranibizumab21 22 and the other two comparing bevacizumab29 30 with PDT treatment. For the RADIANCE and BRILLIANCE study,21 22 results were presented at the end of 3 months because patients in PDT group could receive ranibizumab when needed. A significant increase of BCVA from baseline was observed in both groups. Compared with PDT, the mean improvement of BCVA (WMD=−0.14 logMAR; 95% CI −0.17 to −0.10, p<0.00001, I2=68%; figure 2) and reduction of CFT (WMD=−44.32 µm; 95% CI −59.85 to −28.79, p<0.00001, I2=20%; figure 3) were superior in anti-VEGF group. And the number of patients who gained more than three lines in BCVA was higher in anti-VEGF group (RR=2.42; 95% CI 1.68 to 3.50, p<0.00001, I2=0%; online supplemental figure 1), too. More clinically meaningful VA improvements were obtained with either ranibizumab or bevacizumab treatment. Compared with PDT, patients treated with ranibizumab had a better mean BCVA of −0.13 logMAR and a greater reduction in CFT of 47.89 µm; bevacizumab-treated patients had a better mean BCVA of −0.29 logMAR and a greater reduction in CFT of 24.90 µm (online supplemental material 3, figures 1 and 2).
Supplemental material
Anti-VEGF group recorded two serious ocular AEs (one retinal detachment and one retinoschisis) and PDT group recorded one endophthalmitis (p=0.84; table 2). This endophthalmitis occurred in a patient in the PDT group who received PDT on the first day followed by an injection of anti-VEGF. Therefore, endophthalmitis was considered to be related to anti-VEGF injection. The non-serious ocular AEs showed no evidence of a difference between the two groups (p=0.88; table 2), conjunctival haemorrhage and punctate keratitis were most commonly reported.
Anti-VEGF monotherapy versus PDT combination therapy
Two small RCTs31 32 compared anti-VEGF monotherapy with PDT combination therapy. There was no evidence of differences in mean BCVA (WMD=0.07 logMAR; 95% CI −0.00 to 0.14, p=0.06, I2=61%; figure 2) and CFT (WMD=6.40 µm; 95% CI −20.10 to 32.90, p=0.64; figure 3) between the two groups. The number of patients who gained more than three lines in BCVA (RR=0.92; 95% CI 0.57 to 1.49, p=0.74; figure 3) was similar in both groups, too. Patients in both the anti-VEGF monotherapy group and the PDT combination therapy group obtained significant visual function and anatomic improvements. Nevertheless, the anti-VEGF injections in PDT combination therapy was statistically fewer than anti-VEGF monotherapy group (WMD=1.30; 95% CI 1.24 to 1.37, p<0.00001, I2=32%; table 2). No serious ocular AEs were documented, but some mild non-serious ocular AEs were observed in both groups, including ocular hyperaemia, myodesopsia, conjunctival haemorrhage and eye pain (p=0.22; table 2).
Anti-VEGF retreatment criteria: VA stabilisation versus disease activity
Two RCTs21 22 compared the therapeutic effect of different anti-VEGF retreatment criteria. No evidence of a difference in mean BCVA (WMD=−0.00 logMAR; 95% CI −0.04 to 0.03, p=0.91, I2=0%; figure 2) and CFT change (WMD=2.31 µm; 95% CI −11.46 to 16.08, p=0.74, I2=0%; figure 3) between the two groups. Similar results were obtained for the number of patients who gained more than three lines in BCVA (RR=1.07; 95% CI 0.90 to 1.27, p=0.47, I2=0%; online supplemental figure). Interestingly, the number of anti-VEGF injections guided by disease activity criteria was significantly fewer than in VA stabilisation criteria group (WMD=0.83; 95% CI 0.42 to 1.25, p<0.0001, I2=0%; table 2). The mean change in BCVA (−0.24±0.23 logMA vs −0.24±0.22 logMA) and patients who gained more than three lines in BCVA (47.74% vs 45.00%) from baseline was similar in both anti-VEGF retreatment groups. For anatomical changes, clinically relevant decrease in CFT (−74.72±76.74 µm vs −77.13±97.24 µm) from baseline was observed in both groups.
Safety profile showed no evidence of a difference in patients between the two anti-VEGF retreatment criteria. There were two serious ocular AEs, respective one retinal detachment in VA stabilisation criteria and one retinoschisis in disease activity criteria group. The most commonly reported non-serious ocular AE was conjunctival haemorrhage (p=0.72; table 2).
Discussion
In this meta-analysis, we evaluated the efficacy and safety of anti-VEGF treatment and compared two different anti-VEGF retreatment criteria. Evidences showed that anti-VEGF was superior to improving VA compared with sham or PDT treatment. PDT combination therapy showed similar visual improvement and needed fewer anti-VEGF injections compared with anti-VEGF monotherapy. For different retreatment criteria, anti-VEGF retreatment guided by disease activity criteria could achieve similar visual gain and need fewer anti-VEGF injections compare to VA stabilisation criteria. Therefore, this review can provide the latest update on the systematic review of anti-VEGF treatment and provide evidence for optimising retreatment criteria for myopia CNV.
Myopic CNV was a progressive disease and VA in the sham treatment group became worse than at baseline without treatment.21 The short-term treatment effect of PDT was remarkable, but the long-term effect was poor and the recurrence rate was high.9 13 Analysis results indicated that anti-VEGF therapy had a better visual and anatomical improvement than sham or PDT treatment. The analysis showed that both ranibizumab or bevacizumab improved patients’ VA better compared with PDT treatment. Moreover, the post hoc analyses of RADIANCE study demonstrated BCVA gain of anti-VEGF therapy was sustained over additional 36 months.33
When comparing anti-VEGF monotherapy, PDT combination therapy showed similar visual improvement with fewer anti-VEGF injections. The reduction in the number of anti-VEGF injections may be beneficial for patients who are unwilling or unable to participate in monthly monitoring visits. Patients may also benefit from a reduced risk of complications related to surgery as well as the low-cost benefits of anti-VEGF. Thus, combined PDT with anti-VEGF therapy may be an alternative for the treatment of myopia CNV patients. However, larger comparative studies with longer follow-up are needed to adequately compare the efficacy and cost-effectiveness of anti-VEGF monotherapy with PDT combination therapy.
For safety estimation, there was no evidence of a difference in the incidence of serious and non-serious ocular AEs between anti-VEGF therapy and other treatments. The most common ocular AEs of anti-VEGF treatment were mild conjunctival haemorrhage and punctate keratitis, which were well tolerated in myopic CNV patients. Although some cases reported that new onset myopic macular retinoschisis (MRS) may be a complication of anti-VEGF intravitreal therapy, only one MRS event was reported in MYRROR study, and another study also found there was no association between the new onset of MRS and anti-VEGF therapy.34–36
Currently, the guidance and consensus statement recommended anti-VEGF therapy for myopic CNV, but do not point out the definite criteria for retreatment.12 20 Most clinical research refer to retreatment criteria guided by disease activity criteria (intraretinal or subretinal fluid or active leakage) or VA stabilisation criteria (BCVA change), or both.37–40 The use of different retreatment criteria may affect retreatment rates and the number of anti-VEGF injections. Fewer anti-VEGF injections can lead to lower risk of AEs, preferable compliance and lower cost. Simultaneous monthly measurement of VA stabilisation and disease activity to guide anti-VEGF retreatment are more accurate, but it also imposes a considerable economic burden on health systems. Therefore, it is crucial to determine optimal retreatment criteria, especially for myopic CNV patients in low-income and middle-income countries.41
Two multicentre RCTs21 22 compared different anti-VEGF retreatment criteria for myopic CNV. The results found that disease activity criteria had similar visual efficacy and safety compared with VA stabilisation criteria, but the disease activity criteria required significantly fewer anti-VEGF injections. Analysing the reasons, the anatomical changes that typically precede the actual VA loss, thereby anti-VEGF retreatment guided by disease activity criteria could control disease progression earlier and more sensitive than VA stabilisation criteria.42 43 VA stabilisation retreatment criteria required more frequent injections of anti-VEGF, which means higher treatment costs and increases the possibility of AEs. Thus, anti-VEGF retreatment guided by disease activity criteria may be a more preferred option for the treatment of myopic CNV.
However, there were some limitations in this meta-analysis. The number of included studies was relatively small, and some RCTs had small sample size. There was substantial heterogeneity in some parameters, partly due to inconsistent follow-up times of included RCTs. Besides, the followed-up duration was limited to 12–24 months, which were too short to catch more significant differences in progression of anti-VEGF therapy. Therefore, large, high quality and long-term clinical evidence is needed to support our view in the future.
Conclusions
The meta-analysis suggests that anti-VEGF is effective and well tolerated for improving VA in patients with myopic CNV comparing with sham and PDT therapy. Compared with VA stabilisation criteria, anti-VEGF retreatment guided by disease activity criteria can produce similar therapeutic efficacy and reduce anti-VEGF injections, which may be a more recommended retreatment criterion for myopic CNV patients. Moreover, considering the limitations of the relatively small number and size of studies, it remains uncertain whether the combination of PDT with anti-VEGF therapy can be a good alternative to anti-VEGF monotherapy.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors LD: reviewed literature, data collection, prepared and revised the manuscript. GL: supervision, data collection and data analysis. ZS: data collection and revised the manuscript. XC: data collection and data analysis. JB: data analysis and critical appraisal. CZ: guarantor, supervision, critical appraisal and revised the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.