Article Text
Abstract
Objectives To describe the occurrence of recurrent atherosclerotic cardiovascular disease (ASCVD) events within 3 years after a new-onset event, the associated disease burden and statin prescribing in patients with ASCVD in Taiwan.
Design Retrospective cohort study.
Setting This was a retrospective cohort study using Taiwan’s National Health Insurance Research Database.
Participants In total, 111 399, 133 538 and 21 572 patients who were hospitalised with diagnosis of coronary heart disease (CHD), cerebrovascular disease (CBVD) and peripheral artery disease (PAD), respectively, between 1 January 2012 and 31 December 2014.
Primary and secondary outcome measures For each index and recurrent event, patients were observed for 12 months after admission to quantify risks of mortality, recurrent events, statin treatment and healthcare use.
Results We identified 97 321, 120 914 and 14 794 patients with new-onset CHD, CBVD and PAD, respectively. The proportions of developing first, second and third recurrent events were 22.5%, 25.6% and 30.9% for CHD; 20.9%, 26.2% and 32.4% for CBVD and 40.2%, 41.4% and 43.6% for PAD, respectively. Most patients had the same type of ASCVD for their recurrent events as their new-onset event. The mortality rates increased with each recurrent event (p<0.05 for all three ASCVD groups). The rates of hospital readmission and emergency room (ER) visit increased with increasing recurrent events. For example, in the CHD group, the 1-year readmission rates following the index, first and second recurrent events were 43.1%, 47.6% and 55.3%, respectively, and the proportions of visiting ER were 46.4%, 51.9% and 57.8%, respectively. Statin prescribing was suboptimal at time of index event and recurrent events.
Conclusion Recurrent ASCVD events were associated with a higher risk of recurrent event and mortality and greater healthcare use. However, statin prescriptions at index event and after each recurrent event were suboptimal.
- coronary heart disease
- cardiac epidemiology
- epidemiology
Data availability statement
Data may be obtained from a third party and are not publicly available. The data underlying this article cannot be shared publicly due to ethical and legal restrictions from Taiwan authorities. The data can be accessed by qualified researchers with permission from the Health and Welfare Data Science Center (HWDC), Ministry of Health and available only through HWDC facilities.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This retrospective cohort study using Taiwan’s national health insurance claims data, which provides nationwide estimates of recurrent atherosclerotic cardiovascular disease (ASCVD) events.
The methodology is noteworthy in capturing up to third recurrent ASCVD events and its associated mortality and healthcare use due to its longitudinal study design.
As with all studies using claims data, healthcare uses that are not covered by the national health insurance are not captured in this study.
Introduction
Atherosclerotic cardiovascular disease (ASCVD) comprises acute coronary syndrome, myocardial infarction (MI), unstable angina, coronary or other arterial revascularisation, transient ischaemic attack and peripheral artery disease (PAD).1 Patients who have had a first ASCVD event have an increased risk for future events compared with those with no events.1
Prior studies have indicated that some ASCVD patients develop recurrent events within a relatively short period.2–4 For example, in a US study comprising 48 688 Medicare beneficiaries with index acute MI, the recurrence rate remained relatively high for patients experiencing acute MI or coronary heart disease (CHD), at 68.5 and 124.9 per 1000 person-years, respectively, during 2007–2009.2 In a cohort of 7870 patients with acute MI enrolled in the Osaka Acute Coronary Insufficiency Study, 353 patients (4.5%) experienced recurrent MI with a median follow-up of 3.9 years.3 Another study of 1 96 765 patients with ischaemic stroke in the Swedish Stroke Register (Riksstroke) reported that 11.3% had a recurrent ischaemic stroke within 1 year.5
In addition, as different ASCVD events share common risk factors, such as hypertension, hypercholesterolemia, diabetes mellitus and smoking, some studies have suggested that the recurrent event may not be identical to the index event. For example, 1.4% of patients discharged after acute MI experienced a stroke event during the next 12 months.6 Therefore assessing for other types of recurrent ASCVD events in addition to the index event type may produce a more complete picture of recurrent event risk, but examples of such an approach in the literature are rare.
Although the development of recurrent ASCVD events has been investigated, most existing studies observe event rates and outcomes associated with the first ASCVD recurrence; data on event rates beyond the first recurrence are limited. The epidemiology of ASCVD events and the treatment patterns of these patients are not well understood, particularly in Taiwan. First, it is not known if recurrent ASCVD events incur a higher burden to patients in terms of healthcare use or mortality. Second, it is not clear whether these high-risk patients receive lipid-lowering treatment as recommended by the international guidelines.1 7 Previous studies have suggested that patients with a history of CVD events are often undertreated. Data from Europe show that 80% of such patients are not at low-density lipoprotein (LDL) target, and in low-to-middle income countries fewer than 10% of patients are on multidrug treatment.8
Therefore, the aim of this study is to evaluate the temporal pattern and healthcare burden of recurrent ASCVD events within 3 years following a new-onset ASCVD event (index event) in patients in Taiwan. For each ASCVD event (index, first and second recurrent events), we followed up to 18 months after admission to estimate risk of mortality and recurrent events. Healthcare use was also estimated following each event using the same landmark approach.
Methods
Data source
This retrospective cohort study used data from Taiwan’s National Health Insurance Research Database (NHIRD) from 1 January 2010 to 31 December 2017, provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW). Taiwan’s National Health Insurance (NHI) system is a mandatory, single-payer health insurance programme, which provides comprehensive benefits including inpatient care, ambulatory care, dental care and prescription drugs to its beneficiaries. Over 99% of Taiwan’s 23 million people are covered by the NHI. The NHIRD is a database of uniquely identified claims and transactions for all covered services used by patients enrolled in the programme. It provides patient-level information for research, including demographic, clinical, medical resource use (ambulatory care claims, emergency room (ER) claims and inpatient claims) and treatment patterns. All traceable personal identifiers were encrypted to protect patient confidentiality.9
Study design
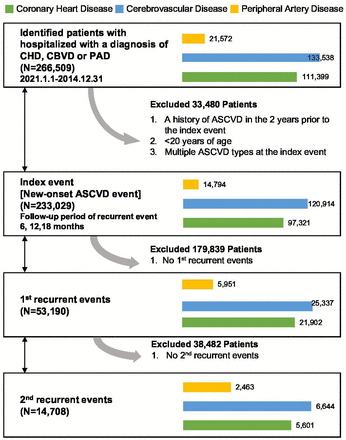
This was a retrospective longitudinal study using Taiwan’s NHIRD. Figure 1 illustrates the study design. We identified patients with a new-onset ASCVD event (index event) and followed up their recurrent ASCVD events (first, second and third recurrent events). The date of the new-onset event was defined as the index date. The 2-year baseline period before the index date was examined to ensure that patients had no history of prior ASCVD and to ascertain baseline characteristics. The observation period for recurrent events was from the index date (inclusive of index date) to death or 3 years after the index date, whichever came first.
Study design. ASCVD, atherosclerotic cardiovascular disease; CBVD, cerebrovascular disease; CHD, coronary heart disease; PAD, peripheral artery disease.
For each ASCVD event (index, first and second recurrent event), we observed patients for 12 months after admission to estimate risks of mortality and recurrent events and healthcare use. Sensitivity analyses using follow-up durations of 6 and 18 months were performed.
Study population
All patients in the NHIRD with a primary ASCVD event during 1 January 2012 through 31 December 2014 and aged 20 years or above were included in this study. An ASCVD event was defined as a hospitalisation with a primary or a first secondary discharge diagnosis of ASCVD. In addition, no history of hospitalisation with any ASCVD diagnosis within 2 years prior to the index event was required to guarantee new-onset events (index event).
We categorised patients with new-onset ASCVDs into three categories using International Classification of Diseases version 9 and 10 (ICD-9/10-CM) codes: (1) those with CHD, including MI (ICD-9-CM codes 410.x, 412; ICD-10-CM codes I21, I22, I25.2), angina (ICD-9-CM codes 411.1, 413.x; ICD-10-CM code I20) and other ischaemic heart disease (ICD-9-CM codes 411.0, 411.8x; ICD-10-CM code I24); (2) those with cerebrovascular disease (CBVD), including ischaemic stroke (ICD-9-CM codes 433.x1, 434.x1; ICD-10-CM code I63) and transient ischaemic attack (ICD-9-CM codes 435.8, 435.9; ICD-10-CM codes G45.0–G45.2, G45.8–G45.9) and (3) those with PAD (ICD-9-CM codes 250.7x, 440.2x, 440.8, 440.9, 443.9, 444.2x, 444.9; ICD-10-CM codes E10.5, E11.5, I70.2–I70.9, I73.9, I74.3, I74.5, I75). Patients diagnosed with more than one type of ASCVD in new-onset hospitalisation were excluded to avoid interaction effects of dual ASCVD events on outcome measurements.
In total, we identified 111 399, 133 538 and 21 572 patients who were hospitalised with a diagnosis of CHD, CBVD or PAD, respectively, between 1 January 2012 and 31 December 2014.
Study variables
Index event and recurrent event
This study identified the index event as the date of new-onset ASCVD events and defined recurrent ASCVD events as occurring within the 3 years of follow-up after the index event (sequentially characterised as a first, second or third recurrent event). Recurrent ASCVD events, defined as a hospitalisation with the primary or the first secondary discharge diagnosis of ASCVD, were also classified as CHD events, CBVD events or PAD events. Length of hospital stay of each event was calculated in days. Time between events was calculated in days between the discharge date of the event and the admission date of the next event. Follow-up time of each event was calculated in months from the discharge of each event until the end of study follow-up.
Risk of mortality and recurrent event
Following each ASCVD event, mortality was identified using the National Death Registry (linked to the NHIRD by encrypted personal identities). Recurrent events were identified using the definition mentioned above. Risk of mortality associated with a recurrent event was followed up from discharge after each event to occurrence of the outcome or 31 December 2017, whichever came first.
Healthcare use
Healthcare use following each ASCVD event (index, first and second recurrent event), excluding use for the event itself, was estimated for 12 months following discharge after the event. Healthcare use was estimated by calculating the number of outpatient visits, ER visits and readmissions. Sensitivity analyses using follow-up durations of 6 and 18 months were performed.
Statin use
Proportions of patients prescribed statins following each ASCVD event (index, first and second recurrent event) were calculated for 12 months following admission for the event. During the study period (2012–2017), statins were used as the major lipid-lowering therapy in the Taiwan NHI system, while ezetimibe was reimbursed when used in combination with statin treatment.10 Therefore, we only described statin use in this study. Sensitivity analyses using follow-up durations of 6 and 18 months were performed.
Statistical analysis
Variables were summarised through descriptive analyses, including tabular and graphical display of mean, standard deviation (SD), median and the interquartile range (IQR) for continuous variables and frequency and percentage for categorical variables.
Following each ASCVD event, risk of all-cause mortality and risk of recurrent event over time were estimated and compared across patients who developed designated subsequent events. For example, among patients with new-onset CHD, the risk of all-cause mortality for the index event was calculated from the time of index event till the end of observation; the mortality risk for the first subsequent event was calculated from the time they developed the first recurrent event till the end of observation, similarly for those developed with the second recurrent event (online supplemental file 1). The data were presented as Kaplan-Meier survival curves. For estimating risk of recurrent events, we conducted Fine and Gray analysis to account for competing risks of death.
Supplemental material
Statistical analysis was performed using SAS V.9.4 (SAS Institute, Cary, North Carolina). Two-sided p<0.05 were considered statistically significant.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Results
Study population
We identified 111 399, 133 538 and 21 572 patients who were hospitalised with a diagnosis of CHD, CBVD or PAD, respectively, between 1 January 2012 and 31 December 2014. We excluded patients with a history of ASCVD in the 2 years prior to the index event and patients younger than 20 years of age. Patients with multiple ASCVD types at the index event were excluded (985 patients in the CHD cohort; 1068 patients in the CBVD cohort and 353 patients in the PAD cohort). Therefore, we analysed the records of 97 321, 120 914 and 14 794 patients with new-onset CHD, CBVD and PAD, respectively (selection flow of study population in online supplemental file 2). Baseline characteristics showed that 68.5%, 59.5% and 57.7% of patients were men, with median age 65, 71 and 74 years in the CHD, CBVD and PAD groups, respectively (table 1).
Patient demographics and characteristics of each event
Index event and recurrent events
Among the new-onset patients, 22.5% of patients with CHD (21 902/97 321), 21.0% of patients with CBVD (25 337/120 914) and 40.2% of patients with PAD (5 951/14 794) had at least one recurrent event (table 1). The proportion of patients developing a recurrent event during follow-up increased with each additional event that occurred (the proportions developing the first, second and third recurrent events were 22.5%, 25.6% and 30.9% for CHD; 21.0%, 26.2% and 32.4% for CBVD and 40.2%, 41.4% and 43.6% for PAD; online supplemental file 3).
With more recurrent events, there was a trend towards a shorter median time to next event in patients with CHD (213 days from index event to first recurrent event; 176 days from first to second recurrent event; 124 days from second to third recurrent event) or CBVD (162 days; 99 days; 72 days), but not in those with PAD (112 days; 112 days; 115 days). The median length of hospital stay showed little change between events in CHD and PAD groups, whereas an increasing trend was observed in CBVD group (from 7 days for index event to 11 days for third recurrent event).
Most patients had the same type of ASCVD for their recurrent events as for their index event; 66%–81% of patients with new-onset CHD had CHD at first recurrence, whereas this percentage was 79%–84% for CBVD and 76%–78% for PAD (online supplemental file 3). When assessing these data by ASCVD type, the proportion of PAD events increased with each additional event in the CHD group (from 6% for the first recurrent event to 19% for the third recurrent event) and CBVD group (from 4% for the first recurrent event to 9% for the third recurrent event). In contrast, the proportions of CHD and CBVD remained stable across recurrences in the non-CHD (CBVD/PAD) and non-CBVD (CHD/PAD) groups, respectively (online supplemental file 3).
Risk of mortality and recurrence rate following each event
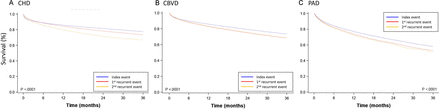
For patients with PAD with one recurrent event, the mortality rate was 40.5%; patients with CHD or CBVD with one recurrent event had mortality rates of 21.8% and 25.8%, respectively. The survival rates decreased as recurrent events accumulated (p<0.05 for all three ASCVD groups; figure 2). The 1-year survival rates following the index event, first recurrent event and second recurrent event in the CHD group were 85.9%, 84.4% and 79.8%, respectively (online supplemental file 4). For patients with CBVD or PAD, 1-year survival rates were highest after the index event, compared with later events, and survival rates were similar following the first and second recurrent event (CBVD: 85.9%, 83.0% and 83.3%, respectively; PAD: 77.0%, 73.7% and 72.5%, respectively.
Survival rates following each event. (A) coronary heart disease (CHD). (B) cerebrovascular disease (CBVD). (C) peripheral artery disease (PAD).
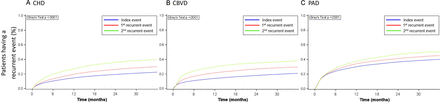
Higher rates of developing another recurrent event were observed when patients experienced more recurrent events (p<0.05 for all three ASCVD groups; figure 3). The 1-year recurrent event rates following the index event, first and second recurrent event were 14.7%, 19.6% and 27.3%, respectively, in the CHD group (online supplemental file 5). A similar trend was observed for the CBVD and PAD groups (CBVD: 14.0%, 21.6% and 29.8%, respectively; PAD: 30.1%, 34.1% and 37.6%, respectively). The risk of having an event increased significantly within the first 6 months after each event (figure 3).
Cumulative recurrent event rates following each event. (A) coronary heart disease (CHD). (B) cerebrovascular disease (CBVD). (C) peripheral artery disease (PAD).
Healthcare use
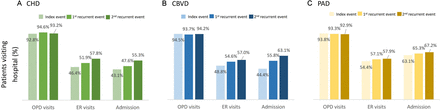
Cumulative rates of hospital readmission and ER visit increased with increasing recurrent events. For example, in the CHD group, the 1-year readmission rates following the index, first and second recurrent events were 43.1%, 47.6% and 55.3%, respectively, and the proportions visiting the ER were 46.4%, 51.9% and 57.8%, respectively (figure 4). The rate of outpatient visits remained over 90% for all patient groups and events. Similarly high rates of healthcare usage were observed even if only hospital visits related to CV health were considered (online supplemental file 6).
Proportion of patients having healthcare use following each event during a 12-month follow-up. (A) coronary heart disease (CHD). (B) cerebrovascular disease (CBVD). (C) peripheral artery disease (PAD).
Statin use
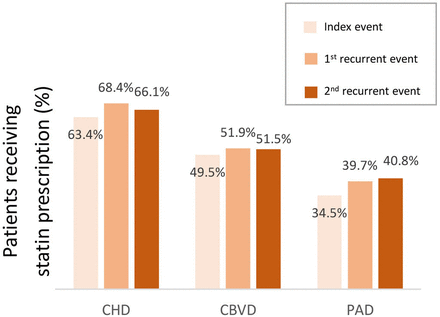
Statin prescriptions after each event were suboptimal in all patient groups: 63.4%–68.4% in the CHD group, 49.5%–51.9% in the CBVD group and 34.5%–40.8% in the PAD group within 12 months following the index event and the second recurrent event (figure 5). Patients with index CHD were most frequently prescribed statins compared with patients with index CBVD or index PAD. Similar trends were evident when assessed at 0–6 or 0–18 months from the event (online supplemental file 7)
Proportion of statin prescription following each event during a 12-month follow-up. CBVD, cerebrovascular disease; CHD, coronary heart disease; PAD, peripheral artery disease.
Discussion
Our study demonstrates a higher risk of recurrence, mortality and increasing healthcare use among patients with occurrence of each additional recurrent ASCVD events; 22.5% of patients with CHD (21 902/97 321), 21.0% of patients with CBVD (25 337/120 914) and 40.2% of patients with PAD (5 951/14 794) at index had at least one recurrence during 3-year follow-up. Patients were more likely to have a recurrent event if they had already experienced a recurrence, and this risk increased with increasing episodes of recurrence. In addition, the study found a trend towards a shorter median time to next event in patients in Taiwan with CHD or CBVD, but a similar median time between PAD events.
A notable finding is the suboptimal prescription of statins among patients in Taiwan with ASCVD events. Only 34.5% of patients with PAD, 63.4% of patients with CHD and 49.5% of patients with CBVD received statins in the 12 months after the index event. This finding is similar to US database studies, where approximately 45% of patients with ASCVD were not on lipid-lowering therapy.11 12 The particularly low statin prescribing rates for PAD patients are also evident in US data; data from PAD patients collected 2005–2012 found only 33.1% were using statins.13 The low statin prescribing rate among PAD patients in our study may contribute to the increased risk of recurrent events in these patients relative to their counterparts with CBVD or CHD. While our data show moderately higher statin prescribing as patients accumulate recurrent events, statin prescribing rates remained suboptimal overall (63%–66% for the CHD group, 50%–52% for the CBVD group and 35%–41% for the PAD group). These data highlight undertreatment in ASCVD management in Taiwan, despite multiple studies confirming that lowering LDL cholesterol with high-intensity statins or PCSK9 inhibitors effectively reduces the risk of primary and secondary cardiovascular events.14–16 Raising local awareness of the recommendations for secondary prevention in international guidelines may help address this problem.
Unlike previous studies,2–5 which only focused on the first recurrent event and a single event type, our study provides a more complete picture regarding the patterns of multiple recurrent events and their associated burden. To the best of our knowledge, our study is the first in Asia investigating the burden of recurrent cardiovascular events. A recent study in Finland has also evaluated multiple recurrent events, including different event types, with some similar findings to our study. In Finnish CVD patients, each additional event caused increased risk of a recurrent event, and the median time of recurrence decreased with increasing numbers of events.17
The pattern of recurrent events showed that patients were more likely to develop the same type of ASCVD in the recurrent events. In our study, 66%–81% of patients with new-onset CHD had CHD at first recurrence, whereas this percentage was 80%–84% for CBVD and 76%–78% for PAD. These findings are in line with a previous real-world study showing survivors of MI and ischaemic stroke are at immediate risk of having an additional cardiovascular event, in most cases of the same type as previously experienced by the patient.18 Nevertheless, our data suggest that around one quarter of patients could experience a recurrent event of a different type to their initial event. Notably, PAD accounted for a larger proportion of recurrent event among patients with index CHD or CBVD event. Therefore, patients receiving treatment for secondary prevention should be educated on recognising signs and symptoms of different types of events, not just their index event.
Our study revealed an increased risk of death with cumulative recurrent events at different follow-up periods (6, 12 and 18 months) across different types of index events; such data are relatively limited in the existing literature.17 18 Furthermore, among all three types of ASCVD we studied, patients with PAD had the highest risk of death and highest incidence of recurrent events with cumulative recurrent events, which is in line with findings from previous studies.19 20 These data indicate an urgent need to improve secondary prevention in patients with ASCVD, especially those with PAD.
There are several limitations to our study. First, since the study used administrative records, we were unable to evaluate healthcare use that was not covered by the NHI, such as out-of-pocket payment. In addition, socioeconomic factor or life style factors (such as diet or exercise) are not available in Taiwan’s national health insurance claims data. Second, our study only focused on recurrent events developing in the 3 years after the index date, and we were unable to capture recurrent events occurring beyond that. Moreover, the risk of mortality and developing recurrent events might be underestimated due to the limited follow-up period after the first and second recurrent event. Third, generalisability of our study may be limited by its study population, as we only included patients with new-onset ASCVD leading to hospitalisation. Therefore, this study is likely to have included patients with ASCVD with higher severity or morbidity. Fourth, as we intended to capture the natural course of recurrent ASCVD events, we did not include other control variables beside age and sex in our competing risk model analyses. Despite the above limitations, the use of claims data from the NHI database in this study provided comprehensive records on ASCVD occurrence, treatment pattern and healthcare use. The database covers over 99% of the population of Taiwan and is representative of Taiwan’s general population; this allowed us to comprehensively investigate patients with ASCVD from the general population in Taiwan.
In a large population of patients in Taiwan, we observed a higher risk of mortality with increasing recurrent events as well as increased risk of developing further recurrent ASCVD events and greater healthcare use, representing an increase in the disease burden. Our data also show suboptimal rates of statin use in these patients highlighting an opportunity to improve secondary prevention in this population.
Data availability statement
Data may be obtained from a third party and are not publicly available. The data underlying this article cannot be shared publicly due to ethical and legal restrictions from Taiwan authorities. The data can be accessed by qualified researchers with permission from the Health and Welfare Data Science Center (HWDC), Ministry of Health and available only through HWDC facilities.
Ethics statements
Patient consent for publication
Ethics approval
This study was reviewed and approved by the Research Ethics Committee of National Taiwan University Hospital (NTUH-REC-201710059W). Informed consent from patients was waived since the data were retrospectively collected and the identification data from NHIRD were encrypted for confidentiality.
Acknowledgments
We thank the National Health Insurance Administration (NHIA) and Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare, for making the databases used in this study available. However, the content of this article does not represent any official position of the NHIA or HWDC. The authors have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Medical writing assistance was provided by MIMS (Hong Kong) Ltd., which was funded by Amgen Taiwan in compliance with Good Publication Practice 3 ethical guidelines (Battisti et al. Ann Intern Med. 2015;163:461–4) and the STROBE checklist for the reporting of observational studies (https://www.equator-network.org/reporting-guidelines/strobe/).
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors C-YH, Wen-JC, H-JL, Wei-JC, Y-HY and F-YH contributed to the study concept and design of the research; C-YH, C-MC and F-YH performed the research; H-MC analysed the data; all authors wrote and approved the manuscript. F-YH is responsible for the overall content as guarantor.
Funding The project was funded by Amgen Taiwan Limited. The title of the project was burden of subsequent cardiovascular event among patients with cardiovascular disease in Taiwan.
Competing interests All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare: Wei-JC and Y-HY are employees of Amgen Taiwan Limited. C-YH, Wen-JC, H-JL, H-MC, C-MC and F-YH received a grant from Amgen Taiwan Limited.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.