Article Text
Abstract
Introduction Cochlear implantation (CI) is a (cost-)effective intervention for people with severe or profound hearing loss. Since its introduction experience increased and the technology evolved, leading to better results and relaxation of CI eligibility criteria. Meanwhile, with national healthcare costs increasing there is a need for evidence of healthcare technology’s value. This protocol describes a study to investigate clinical and participatory outcomes after CI for the currently (expanded) eligible hearing impaired population. The study adds to the current evidence base through its multicentre design, long-term follow-up and use of participatory outcomes alongside standard clinical outcomes.
Methods This multicentre prospective observational cohort study will include at least 156 adult patients with severe-to-profound hearing loss, approximately evenly divided into two groups (1, ages 18–65 years and 2, age >65 years). The measurements consist of audiometry, cognition tests, listening effort tests and multiple generic and disease specific questionnaires. Questionnaires will be administered twice before CI, soon after inclusion at CI referral and shortly before CI surgery, with an annual follow-up of 3 years after CI. The Impact on Participation and Autonomy questionnaire will be used to assess participation. Generalised models (linear, logistic, Poisson) will be used. Mixed effects models will be used to investigate changes over time while exploring differences in subgroups and the influence of covariates.
Ethics and dissemination The study has received ethical approval from the Medical Ethical Committee of all participating centres. The results could provide valuable insights into changes in participatory outcomes of people with severe-to-profound hearing loss after CI. Results will be disseminated through peer-reviewed journals, scientific conferences and professional and patient organisation meetings.
Trial registration number NCT05525221.
- Decision Making
- Adult otolaryngology
- Patient Participation
- Quality of Life
- Otolaryngology
- HEALTH ECONOMICS
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Decision Making
- Adult otolaryngology
- Patient Participation
- Quality of Life
- Otolaryngology
- HEALTH ECONOMICS
Strengths and limitations of this study
Prospectively investigating long-term (participatory) outcomes of cochlear implantation (CI).
Inclusion of a study population reflective of the current eligibility criteria for CI in The Netherlands, strengthening its ecological validity.
This observational study carries confounding risks, as a randomised design was considered neither ethical nor feasible.
Introduction
Unilateral cochlear implantation (CI) is considered effective and cost-effective in rehabilitation of severe-to-profound bilateral sensorineural hearing loss.1–3 Since its introduction experience increased and the technology evolved leading to better results and relaxation of CI eligibility criteria. Meanwhile the healthcare system is under increasing pressure due to increasing national healthcare costs resulting in a need for evidence of healthcare technology’s added value.
Up until now, most studies have investigated the benefits of CI in terms of audiological assessments, pure tone thresholds, speech perception scores and health-related quality of life.4–6 These outcomes generate valuable insights for clinicians, patients and healthcare policymakers. However, there is an additional need for investigations of outcomes that are more closely related to a person’s everyday experience and to investigate the long-term benefits of CI on societal and economic level for persons with hearing loss.7
The outcomes of interest in this study are determined at the intersection between clinical experience, patient’s information needs, research gaps and theoretical frameworks maintained by (inter)national healthcare institutions. The considered theoretical frameworks are the ICF (International Classification of Functioning, Disability and Health (ICF) framework)8 9; Dahlgren and Whitehead model10 11; and the capability approach.12 13 These frameworks and the value judgements inherently involved in selecting outcomes resulted in the set of outcomes that are considered to be of interest to CI stakeholders at various (micro, meso and macro) levels.
At the individual (micro) level, the main interest is in cognition and listening effort. Hearing impairment is associated with a higher risk of cognitive impairment.14 Even though the precise mechanism responsible for this association is not clear, the current understanding that hearing impairment precedes cognitive decline gives reason to assume that interventions in hearing loss might prevent cognitive decline.14 This resulted in an increasing interest in the change in cognitive status post CI surgery compared with pre-surgery. Listening effort is a symptom often mentioned in clinical practice, which resulted in an increasing interest to objectively measure listening effort via pupillometry and the extent to which this is affected by CI.15–17
At the level of the individual in social and community context the focus is on (social) participation, autonomy, communication profile and work experience. Deficits on these dimensions are commonly mentioned by patients in clinical practice and are closely related to a patient’s everyday experience. However, there is still limited evidence regarding the influence of CI on participation, autonomy and work.4 Furthermore, there is a lack of evidence on the influence of CI on communication abilities, which is more broadly defined than hearing abilities assessed by speech perception and audiological thresholds in soundproof booths.4 Gaining more insight in these dimensions could improve preoperative patient counselling regarding expectations and potential benefit of CI.
In the macro socioeconomic domain the focus is on generic and disease specific health-related quality of life, capability, productivity losses, medical consumption and work status. Health-related quality of life of persons with hearing loss and CI recipients is often measured by using the Health Utility Index Mark 3 (HUI3) and Nijmegen Cochlear Implant Questionnaire (NCIQ). Current developments in the field of health economics illustrate an interest in the use of the capability approach which aims to investigate the broader concept of well-being complementary to quality of life.18 19 Only using quality of life measures can lead to an underestimation of the effects of interventions, especially in social care, mental health,20 public health, chronic illness and elderly care.21 Measuring changes in medical consumption and productivity losses or gains yields insights in terms of costs or savings. Using generic tools facilitates comparability across disease areas. The obtained data is informative in itself and can be valuable input for additional health economic analysis to support decision-making.
The objectives of this study are:
To investigate changes during CI waiting time (the time between study inclusion and CI surgery) on the outcomes described above (autonomy; communication profile; participation; quality of life; capability; work; productivity (loss) and medical consumption), in adults with postlingual severe-to-profound sensorineural hearing loss.
To determine the (long-term) developments of these clinical and participatory outcomes and costs for adult CI recipients.
Methods and analysis
This protocol was written by reference to the Strengthening the Reporting of Observational Studies in Epidemiology checklist as applicable to a protocol.22
Patient and public involvement
Healthcare providers and researchers involved the Dutch CI patient organisation Onafhankelijk Platform Cochleaire Implantatie [Independent Platform Cochlear Implantation] (OPCI) and the Dutch National Healthcare Authority in the predesign phase by deliberations regarding main problems in the field of CI. It was collaboratively concluded that it is desirable to gain insights on the aims of this study. These organisations were not involved in the design phase and recruitment. Results will be disseminated via scientific publications, conferences and healthcare professional-organisation and patient organisation meetings, details regarding dissemination will be further specified at the end of the study.
CI trajectory
The CI journey for adults generally starts when a person with severe or profound hearing loss is referred to an audiological centre or a tertiary ENT (ear, nose and throat)-department specialised in CI because the conventional hearing aids do not provide sufficient audiological benefit. At the centre of expertise the patient’s medical history is assessed and hearing tests, like a speech perception tests and pure-tone audiogram, are conducted. If the hearing aid indeed provides insufficient benefit in terms of speech recognition a patient might be eligible for CI. In most Dutch clinics this is a phoneme-score of 70% or less (at 65/70 dB HL, NVA monosyllables). Additional tests will be conducted to verify the CI eligibility and to assess the potential benefit and feasibility. These tests are MRI and/or CT scans, vestibular tests, psychological assessments. Additional information about the rehabilitation is given by the rehabilitation therapist. If the criteria for eligibility are met, the patient will be informed about the positive advice regarding CI. If both the patient and the multidisciplinary CI-team agree on proceeding with CI, the patient will be placed on the surgery waiting list. The waiting time varies within and between clinics, ranging from 0 to 12 months. After implantation there is an intensive rehabilitation period to optimally improve the hearing. Most CI recipients reach stable results after 1-year post surgery. At this moment the rehabilitation phase ends and the life-time follow-up care starts.
Study design
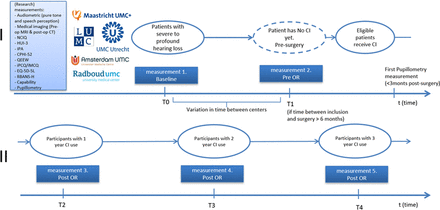
This is a multicentre longitudinal observational cohort study with a follow-up period of 3 years. Two measurements are conducted preoperative (1) short after inclusion, which is as soon as possible after CI referral (baseline) and (2) short before CI surgery if the time between inclusion and surgery is more than 6 months, because we assume no substantial changes in an interval shorter than 6 months. Follow-up measurements are 1, 2 and 3 years post implantation. The study is designed by the ENT department of the Radboudumc in Nijmegen and will be conducted at ENT departments of academic hospitals in The Netherlands, namely: Amsterdam UMC, UMC Utrecht, Maastricht UMC+, Leiden UMC and Radboudumc. The study design and the time of measurements are shown in figure 1.
SMILE study timeline. Questionnaires will be conducted at each time point. Audiometric data will be gathered in line with clinical practice guidelines. Cognition tests will be measured once preoperative and once postoperative if age is <65 years and twice postoperative if age is >65 years. Pupillometry will be conducted only postoperative. CI, cochlear implantation; CPHI, Communication Profile for the Hearing Impaired; HUI3, Health Utility Index Mark 3; IPA, Impact on Participation and Autonomy; NCIQ, Nijmegen Cochlear Implant Questionnaire; QEEW, Questionnaire on the Experience and Evaluation of Work; EQ-5D-5L, EuroQol 5 Dimensions 5 levels; iPCQ, iMTA (Institute for Medical Technology Assessment) Productivity Cost Questionnaire; iMCQ, iMTA (Institute for Medical Technology Assessment) Medical Consumption Questionnaire; RBANS-H Repeatable Battery for the Assessment of Neuropsychological Status for Hearing Impaired Individuals.
Measurements
In addition to the standard clinical examinations, participants will be asked to complete several questionnaires and participate in cognitive and listening effort tests. Figure 1 shows the timeline. Questionnaires will be administered at each time point. The cognition test will be conducted once during waiting time and once 1-year post-surgery if the participant is under the age of 65 years or twice, 1 and 2 years post-surgery, if the participant is over the age of 65. The listening effort assessment (via pupillometry) will be conducted shortly after implantation (±3 months) and 1 year after implantation. These pupillometry measurement time points provide insights into listening effort and how this changes after experience with CI use, alterations in fitting parameters of the speech processor, auditory training as part of the post implantation trajectory, and changes in speech perception.
Study population and recruitment
The following procedures will be followed during the recruitment period (August 2020 till December 2022). The participating clinics invite adult patients who are potentially eligible for CI to participate in the study immediately after being referred to the CI centre. To obtain clear baseline measurements the patient should be included as soon as possible and at least before their final ‘go/no go’ consultation regarding CI. After being fully informed about the study, the CI candidate decides whether he or she is willing to participate in the study. In case of participation the informed consent will be signed.
Incentives
There are no financial incentives for patients to participate in this study. Participants can obtain their personal results on request at the end of the study.
Inclusion criteria
The following inclusion criteria for study participation were applied:
Age 18 years or older. Divided over group 1: age between 18 and 65 years, the working population and group 2: over the age of 65 years, approximately the retired population.
Participants have bilateral severe-to-profound postlingual sensorineural hearing loss (as defined by WHO criteria >61 dB loss) and are being referred to an academic hospital for potential eligibility for CI.
Eligible for CI based on clinical criteria, specifically:
Best aided phoneme score ≤70% at 65/70 dB HL.
Communication need expressed by the patient during CI intake procedure.
Native speakers of the Dutch language.
Exclusion criteria
The following exclusion criteria are maintained for the study.
Patient has an underlying syndrome or psychiatric disorder.
Incapable of performing (un)paid labour, due to non-hearing-related factors.
Prelingual hearing loss.
Children (0–18 years).
Any condition that may hamper a complete insertion of the electrode array or a normal rehabilitation with the cochlear implant (severe otosclerosis or neurological deficits).
Outcome measures
The aim is to gain insight into hearing loss and CI use in the context of everyday life on an individual (micro-, meso-) and societal (macro-) level. Multiple questionnaires and tests will be used to obtain this data. A brief overview of all outcome measures and their domains are presented in table 1.
Outcome measures and their specific subdomains
Primary outcome measures
Participation measured by Impact on Participation and Autonomy questionnaire
Our primary outcome measure is (societal) participation, primarily assessed by using the Impact on Participation and Autonomy questionnaire (IPA). ‘Participation’ in this questionnaire is based on the ICF.23 The IPA is a generic outcome measure, designed to be used with adults with chronic conditions.23 24 First the IPA quantifies the difficulties in aspects of participation and autonomy, by 32 items divided over five subscales.24 The five subscales consist of five, six or seven items. These subscales are: autonomy indoors (seven items); family role (seven items); autonomy outdoors (five items); social life and relationships (seven items); work and education (six items). Second, the IPA evaluates potential experienced problems in participation.25
There are four response options for each of the 32 items about participation and autonomy. These range from ‘very good’ (score=0) to ‘bad’ (score=4).24 There are three response options for the items about problems experienced, ranging from ‘no problem’ (score=0) to ‘big problem’ (score=2). Scores will be summarised per subscale. A higher score indicates more difficulties in participation and autonomy, or more experienced problems with these difficulties. An average of 0 indicates there are no difficulties.24 It is likely that some subscale scores indicate difficulties in participation but simultaneously not perceived as a problem by the participant. For example, because other people adequately compensated in completing this task. At least 75% of the items in each subscale must be answered to yield valuable results.
The IPA subdomains ‘Family role’, ‘Social life and relationships’ and ‘Work and educations’ comprise the used definition of participation. Participation was chosen as the primary outcome since it is considered as valuable across all age categories. Other outcome measures are presented in table 1. A previously conducted cross-sectional pilot study illustrated a difference in participation between CI recipients and hearing impaired individuals waiting for CI (see Statistical analysis paragraph below).
Clinical measures
Clinical outcomes registered in the Electronic Patients Dossier are used in this study and captured in Castor Electronic Data Capture (EDC).26 Audiometric measurements are part of standard CI procedure and are measured by standard audiological equipment. A questionnaire for tinnitus, the Tinnitus Handicap Inventory; pre-implantation and post implantation electronystagmography are also part of standard procedure in all CI patients and used in this study.
Baseline measurement—demographic characteristics
Gender (male/female).
Age (in years).
Duration of hearing loss (years).
Aetiology of hearing loss.
Marital status.
Living situation (living together with a partner, etc).
Level of education (none, elementary and secondary school, after-school training, university, etc).
Occupational status (yes/no).
Ever lost a job due to hearing loss (yes/no).
Country of birth.
Country of birth father.
Country of birth mother.
Hearing loss in each ear: air- and bone conduction thresholds in dB HL measured by four frequency (500, 1000, 2000 and 4000 Hz) pure-tone audiograms (PTA0.5−4kHz), and percentage monosyllabic phoneme recognition, unaided and best aided.
Data collection and management
Following signed informed consent patients will receive a confirmation email with instructions regarding the online surveys which will be sent shortly after (T0). If the time between inclusion and surgery is longer than 6 months, the participant will receive the online survey again 2 weeks prior to surgery (T1). Comparable surveys will be sent each year for a period of 3 years post CI implantation (T2, T3, T4). Compliance will be checked periodically by the researchers. If the participant needs assistance in filling in the questionnaires, or does not have access to the internet, assistance will be provided at the clinic or at home. Only patients included in the Radboudumc will participate in the cognition test (Repeatable Battery for the Assessment of Neuropsychological Status for Hearing Impaired Individuals test battery, see table 1) and pupillometry.
Data will be captured by using Castor EDC. Questionnaires will be administered by using the Castor EDC online survey feature. Paper versions of the questionnaires will be administered on request. Survey reminders will be sent within 14 days after the first invitation. Involved researchers will monitor completion and assess if additional follow-up reminders or phone calls are required. Data from the participant’s medical records, paper versions of the questionnaires and tests will be entered in castor by the involved researchers. Data collection forms and data management plans are available on request.
When a participant drops out, he/she will be removed from the database and mail lists and will no longer receive the surveys. This will likely yield missing values for the analysis. An estimated dropout rate has been accounted for in sample size calculations. Gathered data prior to dropout will be used in the analysis.
Data is handled confidentially. After informed consent, patients will be pseudonymised and the key file is safeguarded by the principal investigator of each research site. Source data can be accessed by the research team. Data will be secured and stored in Castor EDC and in closed rooms with locked cabinets at (restricted access) departments of the participating centres. In line with Good Clinical Practice guidelines data will be stored for a period of 15 years.
Collaboration contracts between the centres were drafted and signed. Each centre has access to Castor data of their included participants. Assigned researchers at the initiating centre will have access to Castor data of all study participants. These researchers will have access to the final data set and lead the analysis and writing of the manuscripts.
Sample size calculation
Sample size calculations are based on the main outcomes for participation, the three subscales of the IPA. Input for these calculations are derived from a pilot study recently conducted in the Radboudumc. In this cross-sectional pilot study, 10 pre-CI patients from the waiting list for CI surgery and 11 adult CI recipients (all with more than 1-year CI experience) provided data on the main outcomes. All participants in the pilot had an age between 18 and 67 years.
Our pilot did not give any insight on correlations between two measurements, which is required to get an idea about the SEs that will be found in the study. We assumed a correlation of 0.5 for any paired observation. An approximation of the SE of a difference between two measurements is then given by sddifference/√n, with sddifference equal to the SD of the difference score and n equal to the sample size. Since we assume a correlation of 0.5 between paired observations, the SD of the difference scores is equal to the (pooled) SD of the two individual scores. The pilot showed differences between the pre-CI and the post-CI groups of −0.57 (pre-CI: 0.71 (SD 0.76) post-CI: 0.14 (SD 0.36)) for family role; −0.79 (pre-CI: 1.3 (SD 0.8) post CI: 0.51 (SD 0.52)) for social life and relationships and −1.26 (pre-CI: 1.9 (SD 1.1) post CI: 0.64 (SD 0.61)) for work and education. The SD ranged from 0.4 to 1.1 with a median value of approximately 0.8. A sample size of 64 would imply a margin of error of 0.2, which is acceptable given the preliminary results of the pilot. Given the patient and study characteristics, the probability of a dropout is considerable so we aim to include 78 patients to accommodate 18% dropout. For group 2, approximately the same number of patients (65+ years) will be included. This group will not provide data regarding work and education, but will provide important data on the outcomes of social life and relationships, autonomy, communication profile, capability and quality of life.
Statistical analysis
Descriptive analyses will be conducted to assess the baseline characteristics. Continuous variables will be summarised with mean and SD or, if not normally distributed median and IQRs, and categorical variables with percentages (numbers).
After visual inspection of the results over time we will fit linear mixed effects models with the subdomains of the IPA as dependent variable and (a function of) time of measurement as independent variable. We will also fit models where we will use T0 to T4 as independent variables. The mapping of time since entering the study to T1 to T4 is an approximation of the precise timing, but these models allow us to more easily verify potential differences in outcome during, for example, the waiting period (T0 vs T1). This model will be extended to find differences in changes among subgroups and investigate the influence of other covariates on the outcome. It is currently difficult to provide a complete list of variables that will be considered as covariates, based on literature some are likely to be explored: age, gender, preoperative speech perception scores, duration of hearing loss and/or duration of hearing aid use, preoperative pure tone hearing thresholds. Furthermore, explorative analysis will be conducted to investigate the influence of waiting time for CI, relatively short versus long waiting times on postoperative outcomes. This might further elucidate the importance of timely implantation.
Ethics and dissemination
Study participants follow current clinical practice, and no additional interventions or invasive tests will be conducted in this study. There are negligible risks involved in study participation. Questionnaires and tests might be perceived as tiresome. This study has been assessed and approved by local medical ethical committees of each participating centre. Major protocol modification will be communicated to the centres and their medical ethical committees, after approval modifications will be applied to the trial registry. Results will be disseminated in peer-reviewed journals, scientific conferences and at professional and patient organisation meetings.
Discussion
For the healthcare system to be sustainable over time and to maintain access to high quality healthcare it is considered important to ensure viability of finances, resources, staffing and societal support.27 There is increasing pressure on these dimensions due to the ageing population, new technologies and an increase in the number of chronically ill people.27 According to the Dutch Scientific Council for Government policy it is important to prioritise and make sharper decisions to achieve sustainable healthcare.27
Continuing (re)evaluation and monitoring of the impact and value of CI, as a specific health technology, is considered important.28 29 In particular because CI eligibility is dynamic and multifactorial and evolved with increased experience and improved technology. As a result CI eligibility criteria changed over the past decades and might shift further in the future. Meanwhile, total healthcare expenditure continues to rise and there is an increasing demand for scarce resources. These developments might contribute to increasing waiting times. Therefore there is a need for justifications and decisions regarding appropriate resource use. From an access to healthcare perspective, (long) waiting times are arguably always undesirable, because of the inherent prolonged period of suboptimal health. Severity of the consequences of waiting time depends on the type of disease and (elective) health service.30 Measuring the consequences of waiting time for specific health domains and interventions might provide insight into the overall burden of waiting and facilitate discussions on priority setting.
This study will gain quantitative insights in participatory outcomes that will be measured with generic tools, for comparability with other disease areas, and with disease specific tools, to ensure sensitivity and to elicit details that might otherwise be overlooked. The results can be used for further deliberation on the impact of severe-to-profound hearing loss and the desirability of CI for hearing rehabilitation in the current eligible population.
This study is distinctive in various ways. The main focus is on measuring changes in an individual’s participatory outcomes, rather than solely audiological outcomes, in adults with bilateral hearing loss who are currently referred for CI and might eventually receive a CI. Furthermore, the timeline of measurements is unique and will elicit additional insights on long-term changes after CI and the developments during waiting time for CI. Participants are included as early as possible after initial referral, with the additional advantage that at baseline assessment participants are not influenced by the anticipation of CI because this is prior to the conclusion of the CI eligibility assessments. The second preoperative measurement aims to investigate changes in waiting time and annual 3-year follow-up measurements to investigate changes over time. This deviates from most studies in which patients are included after being considered eligible for CI and where the study follow-up is often limited to 1 year.
A limitation of this study is the absence of randomisation of the intervention (CI vs no CI) or waiting time (longer vs shorter waiting times for implantation), as it raises ethical concerns. This should be considered during analysis and interpretation of the results. Observational studies are less rigorous than randomised designs to control for extraneous variables. Despite this limitation, the results are considered generalisable for Dutch bilateral hearing impaired individuals who are referred for CI, as five out of eight CI centres in The Netherlands participate in this study.
This prospective multicentre study will give insights into the changes after CI on clinical and participatory outcomes. Results can be of value for citizens, patients, healthcare professionals and healthcare policymakers.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors EAMM and WH initiated the research and conceptualised the study design in collaboration with HGBN, SEK and GJvdW. HGBN further developed the design and was responsible for drafting the manuscript. HGBN, WJH, EAMM and GJvdW worked on the design. HGBN, WJH and EAMM facilitated implementation of the study. Statistical advice was provided by a statistician, ARTD, from the Radboudumc Biostatistics group. In addition, Noud Keijsers (non-author of this manuscript) contributed to the cross-sectional pilot study. HGBN led the writing. EAMM, WJH, SEK and GJvdW were closely involved in the writing process and provided feedback on several draft manuscripts. All authors have read, refined and approved the final manuscript.
Funding Investigator initiated research. Heinsius Houbolt Fund is an ENT-related funding organisation in The Netherlands.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.