Article Text
Abstract
Introduction Children often present to primary care with functional abdominal pain (FAP) or irritable bowel syndrome (IBS), and around half still have abdominal complaints 1 year later. Hypnotherapy is an evidence-based treatment that is used in specialist care, but it lacks evidence in primary care. This study will investigate the (cost) effectiveness of home-based guided hypnotherapy for children with FAP or IBS in primary care.
Methods and analysis We report the design of a pragmatic randomised controlled trial among children aged 7–17 years, diagnosed with FAP or IBS by their general practitioner (GP), with assessments over 12 months. The control group will receive care as usual (CAU) by their GP (eg, communication, education and reassurance), while the intervention group will receive CAU plus 3 months of home-based guided hypnotherapy via a website. The primary outcome will be the proportion of children with adequate relief from abdominal pain/discomfort at 12 months, analysed on an intention-to-treat basis. Secondary outcomes will include the adequacy of pain relief at 3 and 6 months, pain/discomfort severity, pain frequency and intensity, daily functioning and impact on function, anxiety and depression, pain beliefs, sleep disturbances, school absence, somatisation, and healthcare use and costs. We must include 200 children to determine a 20% difference in those with adequate relief (55% control vs 75% intervention).
Ethics and dissemination The Medical Ethics Review Committee of the University Medical Center Groningen, the Netherlands, approved this study (METc2020/237). The results will be disseminated to patients, GPs and other stakeholders via email, a dedicated website, peer-reviewed publications and presentations at national and international conferences. We plan to collaborate with the Dutch Society of GPs to implement the results in clinical practice.
Trial registration number NCT05636358.
- PRIMARY CARE
- Functional bowel disorders
- Paediatric gastroenterology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Early management of functional abdominal pain and irritable bowel syndrome through home-based guided hypnotherapy may prevent symptoms becoming chronic.
Exercises are learnt using digital media (eHealth) and fit well with the target population of young, digitally skilled patients.
Our pragmatic design has high external generalisability and will provide useful information on the (cost) effectiveness of home-based guided hypnotherapy in a real-world setting.
The internal validity may be low due to a lack of blinding, with the potential that children in the control group will seek alternative treatment.
Introduction
Background and rationale
Children often present to primary care with functional gastrointestinal symptoms, such as functional abdominal pain (FAP) or irritable bowel syndrome (IBS), that cannot be explained by an organic condition and risk becoming chronic.1–4 These disorders are associated with reduced quality of life (QoL), school absence, sleep disturbances, anxiety and depression.5 6 However, our limited understanding of their exact pathophysiology and the role of multiple factors in maintaining the complaints can make their management challenging.7 8 Given that secondary healthcare use and parental productivity loss appear to drive the estimated annual healthcare costs of €2512 per child,9 adequate early treatment in primary care could reduce symptoms and the need for referral.
The general practitioner (GP) functions as a gatekeeper to specialist care in the Netherlands, similar to systems in Canada and the UK.10 Therefore, children with FAP or IBS usually present first in primary care, where a GP determines the diagnosis by excluding organic causes based on clinical history and physical examination.11–13 The guideline for FAP published by the Dutch Society of GPs (Nederlands Huisartsen Genootschap; NHG), which recommends good communication, education and reassurance, may not be sufficient for all children.14 15 Around half of these children still report abdominal complaints after 1 year,3 underlining the difficulty of treatment.
Children with FAP or IBS may receive psychosocial interventions in specialist paediatric care due to the strong association between functional symptoms and psychological factors (eg, stress).12 13 15–17 Hypnotherapy is one such option that involves a therapist inducing a hypnotic state by guiding a patient to respond to suggestions.18–20 Studies measuring brain responses in adults with IBS show that hypnotherapy may influence gut motility and normalise visceral sensitivity,21 22 though the mechanisms behind its effect on functional abdominal symptoms are poorly understood. Hypnotherapy is generally considered safe. Limited trials report side effects or adverse events, but some rare, mild to moderate adverse events have been reported.23 Indeed, hypnotherapy should always be performed by a trained professional.24 25 Research in children and adolescents has found that hypnotherapy significantly reduces abdominal pain and symptom scores.18 19 24 Other research in children has proven the non-inferiority of home-based guided hypnotherapy compared with face-to-face therapist-guided hypnotherapy at 12 months (adequate pain relief in 75% and 87%, respectively), though with lower effectiveness among children with long-term symptoms.26 The earlier use of hypnotherapy could maximise its benefits, especially if delivered in primary care. Indeed, home-based guided hypnotherapy could improve how GPs manage children with FAP or IBS, potentially leading to a better prognosis, fewer unnecessary referrals and reduced costs. However, evidence of its (cost) effectiveness in primary care is lacking.
Hypothesis
We hypothesise that, compared with care as usual (CAU) alone, home-based guided hypnotherapy plus CAU will be more (cost) effective for achieving adequate relief from abdominal pain and discomfort in children with FAP or IBS.
Methods and analysis
Study design
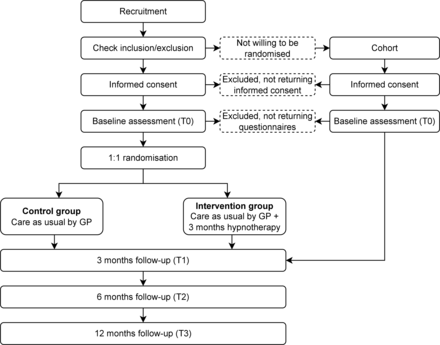
We present the ZelfHy (ZelfHypnose; self-hypnosis) study, a pragmatic randomised controlled trial designed to determine the (cost) effectiveness of home-based guided hypnotherapy plus CAU compared with CAU alone for children with FAP or IBS in primary care. Recruitment has already begun, with eligible children being randomised to either the intervention group or the control group and followed for 12 months (figure 1). This protocol is reported according to the Standard Protocol Items: Recommendations for Interventional Trials guidelines27 and the extended Consolidated Standards of Reporting Trials statement for pragmatic trials.28
Study design. General practitioners (GPs) screen children for eligibility before randomisation to the control and intervention groups.
Study population
The inclusion criteria are as follows: age 7–17 years; attending a GP with chronic gastrointestinal symptoms (eg, recurrent abdominal pain for ≥2 months or ≥2 episodes in the past 2 months); and GP-diagnosed FAP or IBS. GPs base their diagnosis on the following definition: abdominal pain for which the GP does not presume underlying tissue damage, somatic causes, or metabolic or anatomic abnormalities based on medical history and physical examination.14 An overview of the Dutch guideline including definitions of FAP and IBS is shown in online supplemental file 1. Those with a concomitant organic gastrointestinal disease, abdominal symptoms treated by a paediatrician, intellectual disability, psychotic disorders, a history of hypnotherapy in the past year, or poor comprehension of the Dutch language are excluded. Children who prefer not to randomise can choose to enter a parallel observational cohort study in which they complete the same questionnaires.
Supplemental material
Recruitment
We invited GPs either from the Academic General Practitioner Development Network (Academisch Huisarts Ontwikkel Netwerk) or through professional connections. Participating GPs are then asked to recruit study participants during consultations by informing eligible children about the trial and providing written information. Additionally, GP assistants are performing retrospective searches in GP registration databases each month for potentially eligible children, using a search strategy based on International Classification of Primary Care codes (online supplemental file 2). Primary care practices in the Netherlands have been recruiting children since November 2020, and although we had aimed to complete recruitment by September 2022, slow recruitment has necessitated that we extend the end date to September 2023. This slow recruitment by GPs before 1 July 2022 (only 30 children) also led us to expand the routes to participation. Since then, we have now provided information via schools, social media, local media (eg, newspapers and radio) and different interest groups (eg, for parents and IBS groups) to allow self-referral by interested children and/or parents via the study website. The research team then makes contact by telephone, sends the appropriate information and informed consent forms, and asks them to make an appointment with their GP.
Data collection
GPs check the study eligibility criteria using specific forms, irrespective of the recruitment method and send these to the research team. The research team sends the appropriate information and consent forms to children recruited via their GP. A researcher then contacts each child by phone to resolve queries and complete the Rome IV Diagnostic Questionnaire (parent version if <12 years, child version if ≥12 years).29 The research team only sends the baseline questionnaires after obtaining written informed consent from the participant. After receiving the completed questionnaire, they randomise the participant and inform them of their allocation by phone. Follow-up questionnaires are sent at 3, 6 and 12 months. All questionnaires can be completed in around 30 min on paper or via the REDCap (Research Electronic Data Capture) website.30 REDCap sends automatic email reminders after 7 and 14 days if the questionnaires are not completed. After 21 days, researchers remind the participants by phone and ask whether the child has experienced adequate relief from abdominal pain/discomfort (primary outcome). Despite the low risk of (severe) adverse events, we have accommodated spontaneous reporting. All study-related and participant information is stored securely at the study site in locked file cabinets that can only be accessed by researchers.
Randomisation, allocation and blinding
We use a computer-generated 1:1 randomisation list with varying block sizes (4, 6 and 8) and stratification by age (<12 years or ≥12 years). An independent methodologist (M R de Boer, PhD) manages the randomisation list and treatment allocation. The nature of the intervention precludes blinding of the GPs, children and parents, but researchers performing the statistical analyses will be blinded to group allocation.
Intervention
Care as usual
All children receive GP-based CAU according to the NHG guideline for abdominal pain in children,14 which includes communication, education and reassurance. The guideline advocates realistic treatment goals that focus on pain management, rather than pain resolution and appropriate follow-up. Since this is a pragmatic trial, we have not restricted the treatments offered by GPs. Online supplemental file 1 provides an overview of the guideline, which is provided to all participating GPs.
Hypnotherapy by self-exercises
Children in the intervention group receive CAU and are asked to perform home-based guided hypnotherapy for 3 months. Before starting the exercises, a researcher arranges an online video call with the child and parent(s) to explain hypnotherapy, how it can help reduce abdominal pain and how they can access the exercises. Additionally, the child and parent(s) are instructed not to discuss the pain anymore.31
We use an existing home-based guided hypnotherapy programme, as described elsewhere,31 with adjustments. The programme comprises one breathing and progressive relaxation exercise and four visualisation exercises: ‘the favourite place’, ‘the rainbow planet’ or ‘air balloon’ (depending on age), ‘the beach without worries’, and ‘the slide’. Exercises have been recorded by a hypnotherapist in a digital audio format (MP3). Table 1 provides examples of the hypnotic suggestions. The language is adapted to the child’s age, with one version each for children aged <12 years and ≥12 years. Both versions are of equal intensity (eg, exercise duration) and are feasible for all relevant age groups.31 32 We include the instructions and exercises for both versions in a newly designed, responsive, login-protected website. Instructions are directly visible on the home page and vary each week. For example, children are instructed to listen to the first two exercises for the first 2 weeks, with a new exercise introduced every week or 2 weeks. Children can choose what exercise they follow and can repeat it as many times as they want. However, they are asked to listen to the exercises at least five times per week, for 15–20 min per day, over 3 months. To improve compliance, they receive automatic email reminders from the website after 14 and 28 days of inactivity.
Examples of hypnotic suggestions
Outcomes
Table 2 gives an overview of the outcome measures and covariates used in this study. The outcomes are based on a recommended set of variables for clinical trials of paediatric FAP disorders.33 Demographic data are obtained from the inclusion form, and outcomes are measured at baseline and at 3, 6 and 12 months’ follow-up (T0, T1, T2 and T3, respectively). Parents complete the questionnaires for children aged <12 years, while children aged ≥12 years complete the questionnaires themselves, with parental help as needed. Parents always complete the costs questionnaires.
Overview of outcome parameters and covariates
Primary outcome
The primary outcome is the proportion of children with adequate relief of abdominal pain/discomfort at 12 months. The child or parent(s) are asked whether relief from abdominal pain or discomfort has been adequate during the past week, compared with baseline, on a dichotomous scale (yes/no). Self-reported adequate relief is a validated outcome measurement in other trials of IBS treatment.34
Secondary outcomes
Adequate pain relief at 3 and 6 months
The proportion of children with adequate relief of abdominal pain/discomfort at 3 and 6 months will be assessed using the same dichotomous scale as the primary outcome.
Severity of pain/discomfort
The severity of abdominal pain and/or discomfort in the past week is assessed using an 11-point Numerical Rating Scale (NRS-11) from 0 (no pain) to 10 (worst pain). This scale provides valid and reliable scores in children and adolescents with chronic pain.35 36
Pain intensity and frequency
Participants record their abdominal pain or discomfort for seven consecutive days in a diary to aid recall,36 as recommended and often used in other trials of childhood FAP or IBS.31 36 Pain intensity is assessed using an affective facial pain scale,37 38 where the faces range from showing no pain at all (score 0) to the most severe pain (score 3). Pain frequency is assessed by asking how long the pain lasted per day, ranging from no pain (score 0) to >2 hours (score 3). The frequency and intensity scores are then totalled for 7 days, giving ranges of 0–21 per score.26
Quality of life
The KIDSCREEN-52 is a reliable and valid health-related QoL questionnaire that measures the impact of abdominal pain on daily functioning and QoL.39–41 It comprises 52 items covering 10 dimensions: physical well-being, psychological well-being, moods and emotions, self-perception, autonomy, relations with parents and home life, social support and peers, school environment, social acceptance (bullying) and financial resources. Participants rate behaviour frequency or attitude intensity in the past week on 5-point Likert scale. Higher scores correspond to better health-related QoL and well-being.
Anxiety and depression
Symptoms of anxiety and depression are assessed by a short version of the Revised Child Anxiety and Depression Scale (RCADS-25), which is a valid and reliable instrument in Dutch populations.42 The child or parent indicates how often each of the 20 anxiety and 5 depression items applies on 4-point scale from 0 (never) to 3 (always). Higher scores indicate more symptoms of anxiety and/or depression.
Pain beliefs
The paediatric Pain Beliefs Questionnaire (PBQ) includes 32 items that assess beliefs about abdominal pain.43 Each item consists of a pain belief statement with responses ranging from not true at all (score 0) to very true (score 4). The PBQ comprises three subscales: pain threat (20 items), problem-focused coping efficacy (6 items) and emotion-focused coping efficacy (6 items). A higher score on the pain threat scale indicates a stronger belief that their abdominal pain is a threat. Higher scores on both coping subscales indicate stronger beliefs in their ability to cope with pain using problem-focused or emotion-focused strategies.
Sleep disturbances
Sleep disturbances are measured using three items from the Dutch Sleep Self Report questionnaire: ‘Do you fall asleep in about 20 min?’ (score reversed), ‘Do you wake up at night when your parents think you are asleep?’ and ‘Do you feel sleepy during the day?’.44 Children or parents then indicate the frequency in the past week as: rarely (0–1 times), sometimes (2–4 times) and usually (5–7 times). Higher scores indicate more sleep disturbances.
School absence
The cost questionnaire includes an item about school absence in the past 3 months due to abdominal pain/discomfort. Where absence has occurred, they are asked to report the number of days the child actually attended and should have attended.
Somatisation
We use the Children’s Somatisation Inventory to assess somatisation,45 which includes 35 items on physical symptoms. Scores range from 0 (no problems) to 4 (a lot), and higher scores indicate more somatic complaints. The Dutch version has good psychometric properties.46
Cost-utility
The generic EuroQol Five Dimensions Health Questionnaire Youth (EQ-5D-Y) is being used for the cost-utility calculations.47 It contains a descriptive questionnaire and a visual analogue scale. The descriptive system covers five dimensions (ie, mobility, self-care, doing usual activities, pain or discomfort, and emotions). Each dimension is rated on three levels: no problems (1 point), some problems (2 points) and a lot of problems (3 points). Children use a visual analogue scale that ranges from 0 to 100 to rate their overall health (ranging from the worst to the best imaginable health). The EQ-5D-Y is feasible, reliable and valid for children aged 8 years and older.48 Parents of children aged <12 years receive and complete a proxy version of the questionnaire.
Costs
Parents provide information on both medical and non-medical costs using adapted versions of the iMTA Productivity Cost Questionnaire and iMTA Medical Consumption Questionnaire.49 This covers visits to healthcare providers, prescribed medication and hospital admissions, and out-of-pocket expenses (eg, over-the-counter medication, child care, productivity losses and travel costs). In addition, researchers screen the medical records of participating children from 3 months before to 12 months after baseline, seeking to identify the number of GP visits, medication prescriptions, referrals to healthcare providers, hospital admissions and interventions for FAP.
Evaluation of intervention
Usage of intervention
The website is used to collect usage data and measure adherence in the intervention group. This includes the frequency and duration of intervention use (eg, when and for how long children log in) plus data on selected exercises (eg, the exercise chosen and duration). Children are encouraged to attempt the exercises using their own imagination, without listening to the exercises. Children with technical expertise may prefer to listen to the exercises in another way (eg, downloaded). Given that this is not registered on the website, the follow-up questionnaires include an item about whether and how often children performed the exercises without using the website.
Quality of exercises
At 3 months, children in the intervention group rate the quality of each exercise with an overall score from 0 (bad) to 10 (excellent) and describe what they liked about the exercises and what they think could be improved.
Covariates
The prespecified covariates are age (<12 and ≥12 years), baseline pain/discomfort severity and treatment expectations. Children and parents are asked to give their expectations of self-hypnosis by rating whether it will improve symptoms on an 11-point scale from 0 (not at all) to 10 (complete recovery). They are also asked whether they have a (strong) preference for either CAU alone or home-based guided hypnotherapy plus CAU.31
Sample size calculation
We expect adequate relief in 55% of the CAU group and 75% of the intervention group at 12 months,3 26 indicating a required difference of 20% to define treatment success. Therefore, a minimum of 90 children per group will be needed to detect treatment success with 80% power at the 5% significance level. Allowing total loss to follow-up of 10%, we aim to include 100 children per group (200 in total).
Statistical analysis
Clinical effectiveness
We will use appropriate descriptive statistics to describe baseline characteristics in both groups. Estimates of treatment effects (proportions, adjusted mean differences or ORs, as appropriate) will be presented with 95% CIs and p values. All outcomes will first be analysed on an intention-to-treat basis, including all children by the group to which they were randomised. We will then perform per-protocol analyses, including children who did not perform hypnotherapy in the control group and children who started at least four out of five exercises in the intervention group, based on usage data from the website.
The primary outcome will be analysed by logistic multilevel regression modelling, considering relevant covariates. The secondary outcomes will be analysed by logistic (dichotomous variables) and linear (continuous variables) multilevel analyses to investigate the longitudinal relationship between groups. Analysis will be at the patient level for repeated measures in time (baseline, 3, 6 and 12 months), again considering relevant covariates.
Economic evaluation
Costs will be calculated from a societal perspective with a time horizon of 12 months. Healthcare consumption will be assessed based on current Dutch guidelines for economic evaluation,50 calculating the cost for use of the intervention website based on the true resources used. We will perform both cost-effectiveness and cost-utility analyses to compare costs and effects between treatment groups. The cost-effectiveness analysis will include the primary outcome, calculating an incremental cost-effectiveness ratio with the added costs or savings expressed per additional patient with adequate symptoms relief. The cost-utility analysis will use the EQ-5D-Y outcome and express the added costs per additional quality-adjusted life year gained. Finally, we will perform bootstrap re-sampling for both cost analyses to produce confidence intervals, and we will plot cost-effectiveness planes and acceptability curves.
Patient and public involvement
We collaborated with the Dutch Child and Hospital Foundation (Stichting Kind en Ziekenhuis) and incorporated their recommendations in the grant proposal, patient information letters and recruitment strategies. They have also agreed to help disseminate our results to the public. The foundation’s Child Advisory Board evaluated the user experience of the website before it was finalised for the study. Additionally, we asked eight children with FAP or IBS about the primary outcome and used their recommendations to inform our choice of ‘adequate relief’ for this purpose.
Ethics and dissemination
Ethical approval and consent to participate
The Medical Ethics Review Committee of the University Medical Center Groningen has reviewed and approved the ZelfHy study (METc2020/237). Protocol amendments are communicated to the ethics committee and participating GPs as needed. To meet the requirements of Dutch law for medical research (Wet Medisch Onderzoek), participating GPs are asked to agree to study protocol adherence and either parents (age <12 years), parents and the child (age 12–15 years) or the child only (age 16–17 years) are asked to provide written informed consent (online supplemental file 3).
Dissemination
Newsletters concerning study progress and any interim results are being disseminated to participants and participating GPs via the study website and email. The study findings will also be presented at (inter)national conferences and published in peer-reviewed journals, ensuring dissemination of the results to relevant stakeholders, such as GPs (NHG), paediatricians (Dutch Association for Child Paediatrics) and patients (Child and Hospital Foundation, Dutch Digestive Foundation and thuisarts.nl). Study data will be made available on request.
Discussion
The home-based guided hypnotherapy provided in this trial represents an eHealth intervention, delivering or enhancing health services and information through the internet and related technologies.51 Moreover, eHealth for psychological interventions represents an emerging clinical resource when treating children and adolescents with chronic diseases,52–55 proving ideal for use in primary care due to the accessibility and low cost of the exercises. Most adults with IBS prefer remote over face-to-face hypnotherapy, but this has not been studied in children or adolescents.56 A combination of this eHealth strategy with GP communication and education may help empower patients to take control of their own health and learn to manage their symptoms without the help of others.57 58 This strategy supports current efforts to help children with functional complaints learn to manage, rather than completely remove, pain. Despite the expected suitability of our intervention for children with FAP or IBS in primary care, several potential issues warrant further discussion.
The primary outcome could raise questions because the European Medicines Agency and Food and Drug Administration recommend using a NRS as the primary outcome when assessing abdominal pain.59 60 However, these recommendations are based on studies in adults, with none measuring validity or appropriateness for children. Given that there is also a lack of evidence about the optimal treatment outcome in children, we have based our outcomes on recommendations for clinical trials in children with FAP or IBS. These allow the use of an overall measure of change with treatment, a meaningful clinically important difference and a percentage change in symptoms.36 We therefore selected adequate relief as our primary outcome, which corresponds to an overall measure of change with treatment, because treatment in primary care aims to reduce the burden of abdominal pain or discomfort (eg, reducing school absence). Because hypnotherapy aims to reduce both abdominal pain and discomfort, we believe that adequate relief of pain/discomfort is the best outcome measure. Supporting our choice, healthcare professionals, children and parents ranked adequate relief as one of the most important outcome measures.33
This trial benefits from using a pragmatic approach characterised by strong applicability and external generalisability to real-world practice. However, this not only has low internal validity due to the lack of blinding and potential for suboptimal adherence but also precludes aetiological conclusions about the isolated effect of hypnotherapy in primary care.61 62 To increase our understanding for primary care implementation, we plan to supplement this research with a qualitative evaluation of the acceptability of, and facilitators and barriers related to, home-based guided hypnotherapy.
Recruitment according to our initial protocol was hampered by fewer children than anticipated presenting to GPs with functional abdominal complaints, probably due to a higher threshold to see a GP for non-acute complaints during the COVID-19 pandemic. Therefore, we adjusted the recruitment strategy to allow self-referral by children and/or parents. Although this could result in the inclusion of children with less severe complaints, which could in turn influence the primary outcome, we still require that these children visit their GP to optimise comparability. We are keeping track of how patients are recruited to allow later evaluation of differences by the strategy used. Furthermore, we chose to rely on the GP’s assessment of FAP or IBS, in line with current practice in primary care, which also increases the external validity in terms of generalisability. Studies in specialist paediatric care often include children with FAP or IBS based on the Rome criteria, which may differ from our study population. However, by measuring the Rome criteria at baseline, we can evaluate differences between children with and without FAP or IBS according to this standard.
In summary, this protocol describes our approach to study the (cost) effectiveness of home-based guided hypnotherapy for children with FAP or IBS in primary care. In the absence of comparable research in this setting, this study could lead to hypnotherapy being recommended as a supplement to GP-delivered CAU and could improve outcomes for these challenging disorders.
Ethics statements
Patient consent for publication
Acknowledgments
The authors want to thank the Child and Hospital Foundation (Stichting Kind en Ziekenhuis) for helping with the grant proposal, patient information letters and recruitment strategies. Additionally, we thank them for testing the user interface of the website. We also thank Michiel de Boer for performing treatment allocation and providing suggestions concerning the statistical analyses. Finally, we thank Dr Robert Sykes (www.doctored.org.uk) for providing editorial services.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors GAH, MYB, KV, MAB and AMV conceived the original research concept. ING, TF, HJM-A, AH, ALVdV, KV, MAB, AMV, MYB and GAH contributed to the study design. ING and ALVdV are responsible for data collection and management during the trial. ING drafted and revised this manuscript. All authors have contributed important intellectual content to the manuscript and have read and approved the final manuscript.
Funding This work is supported by the Netherlands Organisation for Health Research and Development, ZonMw (grant number 852002035).
Disclaimer The funders have no role in the protocol development and in data collection, analysis, decision to publish or manuscript preparation.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.