Article Text
Abstract
Introduction Atrial fibrillation (AF) is the most common postoperative complication after surgical aortic valve replacement (SAVR) and occurs in up to 50% of the patients. Development of postoperative AF (POAF) is associated with a 2–3 fold increased risk of adverse events, including stroke, myocardial infarction and death.
Several studies have implied that prophylactic Atorvastatin therapy could prevent POAF in patients undergoing coronary artery bypass graft. These studies suggest that Atorvastatin has rapid and significant pleiotropic actions that reduce the risk of POAF. However, prophylactic treatment with statins has yet to be understood in SAVR. The aim of this study is to investigate whether prophylactic administration of torvastatin reduces POAF in patients undergoing SAVR.
Methods and analysis In this investigator-initiated, prospective, parallel-group, randomised, double-blind, placebo-controlled single-centre trial, 266 patients undergoing elective solitary SAVR with bioprosthetic valve, with no prior history of AF, and statin-naïve will be randomised (1:1) to treatment with Atorvastatin (80 mg once daily) or matching placebo for 1–2 weeks prior to and 30 days after surgery. The primary endpoint is POAF defined as an episode of irregular RR-intervals without a traceable p-wave of at least 30 s duration. After discharge and until day 30 after surgery, POAF will be documented by either rhythm strip or 12-lead ECG.
Ethics and dissemination Protocol approval has been obtained from the Regional Scientific Ethical Committee for Southern Denmark (S-20210159), The Danish Medicines Agency (2021103821) and the Data Protection Agency (21/65621).
The trial is conducted in accordance with the Declaration of Helsinki, the ICH-GCP (International Conference on Harmonisation Good Clinical Practice) guidelines and the legal regulations of Denmark. Study findings will be shared via peer-reviewed journal publication and conference presentations.
Trial registration number NCT05076019.
- CARDIOLOGY
- Echocardiography
- Pacing & electrophysiology
- Valvular heart disease
- Cardiac surgery
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Strengths include the investigator-initiated, stratified randomisation and double-blind placebo-controlled design.
The study investigates statin-naïve population without pre-existing atherosclerotic disease.
This study does not include continuous rhythm data after discharge.
Introduction
Atrial fibrillation (AF) is the most common postoperative complication after surgical aortic valve replacement (SAVR) and associated with substantial increases in mortality, morbidity and costs.1–3 Postoperative AF (POAF) occurs in 17%–50% of patients after cardiac surgery and is associated with: a 2–3 fold increased risk of adverse events, including stroke, myocardial infarction (MI) and death; a 4–5 fold increased risk of recurrent AF within the next 5 years; prolonged time in intensive care unit (ICU) (by 12–24 hours) and hospital stay (average of 5 days); and a significant increase in hospital costs.4–8
The mechanisms of POAF are incompletely understood. Intraoperative and postoperative factors, such as course of surgery and postoperative remodelling processes, seem to affect a vulnerable atrial substrate created by pre-existing factors.7 9 Several pathophysiological mechanisms have been described in POAF, for instance, peak inflammatory state, sympathetic activation, autonomic dysfunction and oxidative stress3 5 10 11 but not in all studies.7 Furthermore, inflammation in atrial tissue is described as a risk factor for POAF,7 and it is well known that inflammatory signalling pathways have a causal role in the development of AF. Studies have demonstrated that some cytokines can serve as biomarkers to predict the incidence of AF including C reactive protein (CRP), TNF- α, interleukin(IL)-6, IL1 β, IL-8 and IL-10.12 These markers are secreted by epicardial adipose tissue affecting the atrial myocardium by facilitating arrhythmogenesis.13 Similar findings have also been made in POAF, and biomarkers such as CRP, IL-2 and IL-6 have been significantly increased, but not in all investigations.7 POAF may, therefore, be prevented by targeting inflammation, oxidative stress and autonomic alterations.
POAF and anti-inflammatory properties of statins
Studies on the prevention of POAF with anti-inflammatory drugs including statins have demonstrated conflicting results.3 7 14 15
The specific mechanism by which statins affect POAF has not been thoroughly characterised. However, statins have several well-known pleiotropic effects with anti-inflammatory properties.16 17 These properties are elucidated by reducing adhesion molecules and chemoattractant proteins,18 proinflammatory transcription factors (NF-κB), proinflammatory enzymes and blood biomarkers of inflammation including CRP.19 It is important to state that these properties vary between the different statins drugs with Atorvastatin (lipophilic) showing the most consistent results.18
Some studies of statin treatment showed a reduction of the incidence of POAF after cardiac surgery20 21 as well as moderating myocardial injury (ie, creatine kinase MB (CKMB), troponin I or T, myoglobin and CRP), with perhaps a favourable effect on left ventricular ejection fraction (LVEF), and shorter length of stays in the ICU and in hospital.22–25 However, inconsistent data exist including an increased risk of acute kidney injury.15
Statin treatment in patients undergoing SAVR
Several studies have tried to find prediction models for POAF to identify the patients at highest risk to start prophylactic treatment, but so far, no study has found a reliable multivariable risk prediction model, while only age and male sex are consistent findings.3 7 8 26–28
Beta-blockers, amiodarone and to some extent statins have demonstrated an effect on the prevention of POAF,29 30 however, they are still not recommended as standard.4 The majority of studies on the pharmacological prophylaxis of POAF are conducted in patients undergoing coronary artery bypass grafting,22 29 31–34 and their results should not be extrapolated to patients undergoing other cardiac surgical procedures (ie, aortic valve replacement).25 This is especially important for amiodarone, as patients undergoing aortic valve procedures are more prone to developing atrioventricular block, in which amiodarone has shown to increase the risk of torsade de pointes.35
In the present trial we will, therefore, focus on patients undergoing SAVR (with aortotomy) and the development of POAF. The aim of our trial is to investigate whether a longer perioperative prophylactic treatment with Atorvastatin can prevent the development of POAF and other postoperative complications in these patients.
Hypothesis
This clinical trial will test the hypothesis that administering Atorvastatin 80 mg once daily, from 7 to 14 days preoperative until 30 days postoperative, reduces the incidence of POAF in statin-naïve patients undergoing elective solitary SAVR with bioprosthetic valve.
Methods and analysis
Trial design
The study is an investigator-initiated, prospective, parallel-group, randomised, double-blind, placebo-controlled single-centre trial. This study followed the Standard Protocol Items for Randomised Trials statement.36
Participants
Inclusion criteria
Patients undergoing elective solitary SAVR with bioprosthesis.
Patients who are in sinus rhythm and not taking any antiarrhythmic medication, other than beta-adrenergic blocking agents, at the time of surgery.
Statin-naïve (no prior use of β-hydroxy-β-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors the last 3 months and at least consecutive 7 days prior to the time of surgery).
Age >60 years.
Willingness to participate and provision of informed consent.
Exclusion criteria
Prior history of AF.
Prior history of cardiac surgery.
Known adverse reaction to HMG-CoA reductase inhibitors.
Hepatic dysfunction (alanin-aminotransferase (ALAT) more than twice the upper limit).
Creatinine >200 µmol/L.
Known intolerance to statins or history of muscle toxicity with statins.
Known intolerance to any of the excipients in Lipistad.
Treatment with antiviral medicine (glecaprevir/pibrentasvir) for hepatitis.
Intervention
Patients will be randomly assigned in a 1:1 fashion to Atorvastatin (80 mg once daily) or matching placebo 7–14 days prior scheduled SAVR until 30 days postoperative. Compliance will be documented with a picture of the remaining tablets to account for excess medication. Time schedule of enrolment, interventions, assessments and visits for participants is presented in figure 1.
Timing and frequency of patient–physician interactions and investigations.
Outcome
POAF (I48) is defined as irregular RR-intervals without a traceable p-wave before each QRS complex during at least 30 s or entire 12-lead ECG in symptomatic or asymptomatic patients with no prior history of AF or flutter are considered.4 Continuous ECG monitoring (eight-lead ward monitor) will recognise AF during the entire hospitalisation. Anamnesis, electronic health record or confirmatory rhythm strip or 12-lead ECG of AF until 30th postoperative day are also considered as POAF.
We will also report the burden of AF, and treatment with a rate controlling drug, antiarrhythmic drug, or electrical cardioversion.
Primary endpoints:
In-hospital incidence of POAF
Thirty-day incidence of POAF
Total duration of POAF episodes experienced in-hospital (unit: hours)
Secondary endpoints:
Death
AF: I48 after 30th postoperative day
Plasma-Atorvastatin
MI: I21
Stroke: I60, I61, I62, I63, I64, I69
Trans ischaemic attack: G45
Heart failure: I50
Renal failure: N17, N18, N19
Permanent epicardial pacemaker (KFPF, BFCA)
Wound complication after median sternotomy (superficial or deep infection)
Re-exploration for bleeding within 24 hours
Reoperation for valve dysfunction or ischaemia
Respirator use more than 2 days
New-onset dialysis
Postoperative mechanical support (Left Ventricular Assist Device, Biventricular Assist Device, Intra-Aortic Balloon Pump, Extracorporeal Membrane Oxygenation)
Coronary angiography
Reoperation for tamponade
Pleuracentesis
Pericardiocentesis
Antibiotic treated infection
Echocardiography assessed differences
Length of stay on ICU and in hospital
Rehospitalisation due to infection or POAF
Quality of life (EuroQol-5 Domain, EQ-5D)
Echocardiography
Prospective patients will have performed a comprehensive Doppler echocardiography using a GE medical S70 ultrasound system. Images will be obtained from the parasternal and apical windows. Pulsed Doppler measurements of mitral inflow will be obtained with the transducer in the apical four-chamber view, with a 1–2 mm Doppler sample volume placed between the tips of mitral leaflets during diastole. Tissue Doppler imagining of the mitral annulus will be obtained from the apical four-chamber view with a 1.5 mm sample volume placed at the medial and lateral mitral annulus. All Doppler echocardiographic examinations will be done with horizontal sweep set to 100 mm/s. At least 3–5 cardiac cycles will be measured. For speckle-tracking analyses frame rate will be kept as high as possible with a minimum rate of 70 /s.
LA volume will be estimated using the biplane area length method, with the use of two orthogonal apical views. LA volume will be corrected for body surface area.
Maximal (LAmax) and minimal (LAmin) left atrial volume will be measured at end-systole and the first frame after mitral valve closure, respectively, with the use of two orthogonal apical views. LA reservoir function will be calculated as LAmax-LAmin.
From two-dimensional (2-D) images, systolic longitudinal and radial myocardial deformation (global strain and systolic strain rate) will be assessed using automatic tracking of movement of speckles. Global longitudinal strain will be measured as the magnitude of strain at the aortic valve closure; longitudinal systolic strain rate (SRs) as the maximal negative SR value during the ejection fase. Both parameters will be assessed in all three apical planes and the mean values (GLSmean, SRmean) will be calculated
From 2-D images, the averaged apical and basal rotation will be used for calculation of LV twist and torsion.
End-systolic, end-diastolic volume and LVEF will be calculated according to the Simpson modified biplane method.
LV mass will be estimated using the recommendations of the European and American Society of Echocardiography.37
From the pulsed wave mitral inflow signal, peak E wave velocity, peak A wave velocity and mitral E-wave deceleration time will be measured.
From the tissue Doppler assessment of the medial and lateral mitral annulus early (e') diastolic velocity will be recorded. Diastolic function will be graded in grades 0–3 according to guidelines.
From peak tricuspid regurgitant velocity and size of inferior v. cava pulmonary arterial systolic pressure will be estimated.
Sample size
Systematic literature review was conducted of randomised, controlled studies with planned preoperative treatment for ≥5 days with a sample size ≥100.22 31–33 Absolute risk reduction rates (ARR) of POAF were 17%–22.9% and an RRR of 30%–58%. We recently discovered that the incidence of POAF was 36% in Denmark after SAVR with bioprosthetic valve.38 We have chosen a conservative ARR of 16% and an RRR of 44.4% leaving us with an expected incidence of POAF at 20%. We used Fisher’s exact two-sample proportions test to calculate the sample size; incidence in placebo group 36%, incidence in statin group 20%, alpha 0.05, beta 0.2, power 0.8 and enrolment ratio 1:1, leading to the inclusion of 133 experimental subjects (Atorvastatin 80 mg) and 133 control subjects (placebo).
Stratified randomisation, allocation
Subjects will be randomised 1:1 between Atorvastatin (80 mg once daily) or matching placebo stratified for beta-blockers, sex and age group (60–65, 65–75 or ≥75). Randomisation will take place 7–14 days prior to planned SAVR. We used concealed allocation with varying block sizes (4, 6 and 8) for balanced group assignment.
Blinding
A unique code will be generated for all patients to allow for data management. The list of patients enrolled in the trial is available to the data and safety monitoring board. Patients, physicians, nurses and other data collectors are kept blinded to the allocation during the trial. The placebo is allocated in identical non-transparent containers and resembles the investigational medicinal product for taste, smell, colour and shape. The trial will be unblinded when the last patient has been followed for at least 30 days.
Statistical methods
All analyses will be done according to the intention-to-treat principle. Primary and secondary outcomes will be assessed by calculating the incidence and HR of event among statin-allocated patients and among placebo-allocated patients. Time-to-event analyses will comprise non-parametric Kaplan-Meier plots as well as log-rank tests and semi-parametrical Cox Proportional Hazards regression. In case of competing risks analysis, the Nelson-Aalen estimator for cumulative incidences will be employed. Analyses of length of stay on ICU and in hospital will comprise both a statistical test (the log-rank test) for the difference in discharge time between groups as well as estimates of the median time to discharge (with respective 95% CIs).
Baseline characteristics for quantitative variables will be described by mean and SD or range (minimum-maximum), depending on the shape of the distribution as judged by histograms with approximating normal distributions. For qualitative variables, proportions and respective percentages will be shown. Intergroup comparisons will accordingly be done with t-tests or one-way analysis of variance (alternatively: Wilcoxon rank sum test or Kruskal-Wallis test) and χ2 test (alternatively: Fisher’s exact test), respectively.
Missing data will be clearly reported, and multiple imputation will be used for sensitivity analysis if more than 5% of expected data points will be missing.
Point estimates will be supplemented by respective 95% CIs where appropriate. A p<0.05 will be considered statistically significant. Statistical analyses will be performed with STATA/IC V.17 (StataCorp) and RStudio Team (2020, RStudio: Integrated Development for R. RStudio, PBC, Boston, Massachusetts, USA, URL http://www.rstudio.com/).
Patient and public involvement
The Regional Scientific Ethical Committee for Southern Denmark, on behalf of the patients and relatives, has approved the study and the written participant information and endorsed the ethical perspectives.
Organisation
Patients are assessed for solitary SAVR at a multidisciplinary team conference (with attendance of cardiologist, cardiac surgeons and anaesthesiologist) based on clinical evaluation, echocardiographic ultrasound, coronary angiography and lung function test. Potentially eligible patients will be prescreened according to inclusion/exclusion criteria at the time after eligibility of surgery.
Patients eligible to participate will be presented with the information of the trial at the time of their outpatient appointment, according to normal routine at Odense University Hospital. At this appointment, the patient receives a physical examination, and a cardiac surgeon obtains the journal record (approximately 7–14 days prior to planned surgery).
Subjects who have provided signed informed consent will be randomised. The patient consent form is available in online supplemental file 1.
Supplemental material
During the study, the GCP-monitor, the Scientific Ethical Committee and the Danish Medicines Agency will have access to the complete database including the randomisation list on request.
The data registration is performed via Research Electronic Data Capture with logging and secure storage directly on a server under Odense Patient data Explorative Network, Region of Southern Denmark.
The Executive Committee and Steering Committee consists of LK, LR and PEM (Department of Cardiothoracic and Vascular Surgery, OUH), AB, JSD and MA (Department of Cardiology, OUH) and OG (Department of Nuclear Medicine, OUH) will handle the decisions regarding the overall organisation including administration, budget and use of the database. All practical issues concerning the treatment and data sampling will be handled by the steering committee. The data and safety monitoring board consists of the following experts: LR, LK and PEM (Department of Cardiothoracic and Vascular Surgery, OUH), AB and JSD (Department of Cardiology, OUH), who all have large experience in conducting randomised clinical trials.
Interim analysis
One member of the data and safety monitoring board (LK and a consulting statistician (OG) will evaluate the primary endpoint after 3 years of data collection on the primary endpoint with 30-day follow-up. This interim analysis will comprise 60% of the patients to be included (ie, 0.6×266=160 patients), and the primary hypothesis will be tested conservatively applying an O’Brien-Fleming type α-spending function (ie, 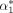 ), resulting in a significance level of 0.0114 at interim and securing an experiment-wise type 1 error of maximal 0.05.39
), resulting in a significance level of 0.0114 at interim and securing an experiment-wise type 1 error of maximal 0.05.39
Ethics and dissemination
The foreseeable risks, side effects and disadvantages have been carefully weighed against the benefit and are not assessed as unjustifiable based on the absolute and the relative risk. The risk of ordinary side effects of Atorvastatin (80 mg orally daily) with no dangerous outcome are 1%–10% corresponding to 13 patients in our trial. These include diarrhoea, flatulence, nausea, constipation, liver effects, allergic reactions, elevated plasma creatine kinase, hyper glycaemia, arthralgia, muscle cramps, myalgia, back pain, pain in extremities, headache, epistaxis, pharyngitis and rhinitis. The risk of uncommon and rare side effects including rhabdomyolysis and pancreatitis are 0.01%–0.1% and <0.01% corresponding to <0.1 patient in our trial.
The use of Atorvastatin/placebo arms with 1:1 allocation is necessary due to the trial design, as it a randomised, double-blind, placebo-controlled, clinical trial. Subjects are not at risk of serious or irreparable harm. The 7–14 days plus 30 days of trial period is the minimal time period based on statistical and clinical considerations.
The restrictive trial criteria and the telephone calls help to ensure, consideration for the subject’s safety, rights and well-being takes precedence over scientific interest. We provide a telephone hotline 24/7, where patients can contact the trial investigator, sponsor or investigator, including handing out a card on which the contact and trial information appears, so that the trial participant and health professionals can contact the sponsor or investigator in case of questions. On the basis of the present, it is assessed that the expected gain in therapeutic terms may justify risks in the trial.
The trial will be conducted in accordance with the Declaration of Helsinki, the ICH-GCP guidelines and the legal regulations of Denmark. The protocol including the informed consent form is approved by the Regional Scientific Ethical Committee for Southern Denmark (S-20210159), The Danish Medicines Agency (2021103821) and the Data Protection Agency (21/65621). The study is registered at ClinicalTrials.gov (NCT05076019). It is also registered in EudraCT (2021-002210-13) as the first out of two subtrials, which are analysed independently. The second subtrial is an investigation in non-statin-naïve patients.
Data will be processed confidential and secure in accordance with the EU General Data Protection Regulation under the Danish Data Protection Act. The study did not start prior to written approval from these institutions.
Project results reporting the primary endpoint will be published in peer-reviewed international journals. The impact of Atorvastatin on the development of POAF will be published, regardless of positive, negative or inconclusive results. Advanced echocardiographic analysis will be published separately.
The order of the authors will be LK, AB, PEM, JSD, OG, MA and LR. Furthermore, the trial results will be submitted to EudraCT within 1 year after the experiment has ended according to guidelines.
The study findings will also be disseminated through presentations at relevant national and international conferences.
Discussion
Randomised controlled studies with planned preoperative treatment for ≥5 days with a sample size ≥100 observed an ARR between 17%–22.9% and a relative risk reduction rate (RRR) between 39% and 58%.22 31–33 Other studies suggest that statin therapy may not be effective in preventing POAF or perioperative myocardial damage, and could potentially increase the risk of acute kidney injury.15 23 40–44 We hypothesise that the inconsistent results may be attributed to factors such as varying treatment durations, the absence of continuous ECG monitoring for AF assessment or a significant increase in type 2 error. In the well-designed STICS trial15 (a randomised, placebo-controlled trial including 1922 patients scheduled for elective cardiac surgery), Rosuvastatin 20 mg/day did not reduce the incidence of POAF (OR 1.04, 95% CI 0.84 to 1.30), despite attenuation of the inflammatory response. However, 34% (653 out of 1922) of the population was not statin-naïve when enrolled in the trial, and only 23% (449 out of 1922) of the population received the assigned regimen 4–8 days before surgery. Furthermore, proinflammatory cytokines are secreted in atrial epicardial adipose tissue, and Rosuvastatin is lipophobic why the difference of anti-inflammatory properties of lipophilic statins versus hydrophilic statins remains yet to be answered.
We thus propose that statin-naïve patients with a plasma concentration of the lipophilic Atorvastatin above a certain threshold on the day of surgery may have a reduced risk of developing POAF. Our definition of AF is according to guidelines.4 The definition, however, varies between studies. We will, therefore, also report the burden of AF in all patients and treatment, which will permit comparable analyses to other studies.
The anti-inflammatory properties of Atorvastatin may be more important in the acute perioperative phase that during long-term treatment.45 46
Taken together, Atorvastatin may have rapid and significant pleiotropic actions that have potentially important (but not proven yet) implications for POAF and cardiac protection in patients undergoing aortic valve surgery.
Applied tests during the study
Medical interview
After 30 days (−0/+7 days) postsurgery, an interview is conducted by a physician and evaluated the following: incident of AF-related discomfort and contact with physicians or hospitalisation, other cardiovascular disease, dyspnoea and quality of life (including EQ Group EQ-5D).
Laboratory assessment
Blood samples are obtained at baseline and during hospitalisation (see figure 1).
Routine parameters: Troponin-T, CKMB, CRP, leucocytes, haemoglobin, platelet count, creatinine, ALAT, HbA1c.
Plasma-torvastatin concentration.
Plasma-Atorvastatin concentration measurements will be revealed after unblinding of the study.
Ethics statements
Patient consent for publication
Acknowledgments
Data management and statistical advice was provided and REDCap was hosted by OPEN, Open Patient data Explorative Network, Odense University Hospital, Region of Southern Denmark.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors LK, LR, AB, OG, JSD and PEM conceived and designed the study. LK and LR wrote the study protocol. AB, OG, JSD, PEM and MA thoroughly revised and edited the protocol. LR is sponsor and principal investigator. LK is coinvestigator. LK is the PhD fellow responsible for the management of the clinical trial. JSD, MA and LK are responsible for the echocardiography protocol. OG and LK are responsible for statistics. All authors have participated in the writing and revising of the manuscript.
Funding University of Southern Denmark, PhD Scholarship, DKK 430.324 (Grant # N/A). The Region of Southern Denmark Ph.d.- Scholarship 2021—2. round—A1126, PhD Scholarship, DKK 584.00. This work is part of a PhD project. The first author received a PhD scholarship from Regional of Southern Denmark PhD Fund, at Regional of Southern Denmark, Denmark and the University of Southern Denmark.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; internally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.



