Article Text
Abstract
Objective To estimate the cost-effectiveness of running a paediatric oncology unit in Ethiopia to inform the revision of the Ethiopia Essential Health Service Package (EEHSP), which ranks the treatment of childhood cancers at a low and medium priority.
Methods We built a decision analytical model—a decision tree—to estimate the cost-effectiveness of running a paediatric oncology unit compared with a do-nothing scenario (no paediatric oncology care) from a healthcare provider perspective. We used the recently (2018–2019) conducted costing estimate for running the paediatric oncology unit at Tikur Anbessa Specialized Hospital (TASH) and employed a mixed costing approach (top-down and bottom-up). We used data on health outcomes from other studies in similar settings to estimate the disability-adjusted life years (DALYs) averted of running a paediatric oncology unit compared with a do-nothing scenario over a lifetime horizon. Both costs and effects were discounted (3%) to the present value. The primary outcome was incremental cost in US dollars (USDs) per DALY averted, and we used a willingness-to-pay (WTP) threshold of 50% of the Ethiopian gross domestic product per capita (USD 477 in 2019). Uncertainty was tested using one-way and probabilistic sensitivity analyses.
Results The incremental cost and DALYs averted per child treated in the paediatric oncology unit at TASH were USD 876 and 2.4, respectively, compared with no paediatric oncology care. The incremental cost-effectiveness ratio of running a paediatric oncology unit was USD 361 per DALY averted, and it was cost-effective in 90% of 100 000 Monte Carlo iterations at a USD 477 WTP threshold.
Conclusions The provision of paediatric cancer services using a specialised oncology unit is most likely cost-effective in Ethiopia, at least for easily treatable cancer types in centres with minimal to moderate capability. We recommend reassessing the priority-level decision of childhood cancer treatment in the current EEHSP.
- Health economics
- Health policy
- Paediatric oncology
Data availability statement
Data are available upon reasonable request. The data sets used and/or analysed in the current study are available from the corresponding author upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The cost-effectiveness analysis was informed by robust primary costing data from Tikur Anbessa Specialized Hospital.
We mitigated the lack of local data on childhood cancer survival rates by conducting a scoping review.
The model does not capture the heterogeneity of childhood cancers, such as variation in cost of care, treatment duration and diverse clinical scenarios, including survival rate.
Background
Globally, childhood cancer (age 0–19 years) represents 0.5%–4.6% of the total cancer burden in a population,1–4 and nearly 90% of this burden falls on low and middle-income countries (LMICs).5–7 In 2017, childhood cancer represented a disease burden of 11.5 million disability-adjusted life years (DALYs) globally and ranked as the sixth and ninth leading causes of disease burden in total cancer and childhood disease, respectively.8 Over the past few decades, high-income countries have dramatically improved the treatment outcomes of childhood cancers. In the UK, for example, the 5-year survival rate has increased from less than 30% in the 1960s to almost 80% on average in the 2000s.9–13 By contrast, survival rates in Africa generally remain lower than 20%,7 14–16 and these avoidable deaths are largely due to late diagnosis, misdiagnosis, lack of access to quality therapeutic and supportive care, high treatment abandonment rate, treatment adverse effects and avoidable high rate of relapse.14 17
In general, there is a significant lack of reliable data on the disease burden of childhood cancers in Ethiopia. The latest estimates from GLOBOCAN 2018 put the incidence of cancer among children aged 0–14 at 3800 cases annually, or 8.9 per 100 000 children.2 3 Another study on cancer incidence in Ethiopia estimated 3707 annual cases as of 2015.18 The most common childhood cancers in Ethiopia are acute lymphoblastic leukaemia (25.7%), non-Hodgkin’s lymphoma (8.9%), rhabdomyosarcoma (8.9%), Wilms tumour (8%) and neuroblastoma (7.8%).19 20
Sadly, as in other low-income countries (LICs), most childhood cancers in Ethiopia are not successfully treated. One Ethiopian study examined all children below 15 years of age admitted to the paediatric wards of Gondar University Hospital due to cancer in 2010–201321 and found that only 20% improved, while 65% were discharged without improvement and 7% died in the hospital. The main reason for discharge was the unavailability and unaffordability of chemotherapeutic drugs. In addition to the challenge of obtaining supplies and the unaffordability of treatment, there is also a large gap in the availability of equipped facilities and trained staff. As of 2019, Ethiopia had only six qualified paediatric hemato-oncologists for the entire nation,19 and access to diagnostic or treatment centres is very limited. Until recently, Tikur Anbessa Specialized Hospital (TASH) had the country’s only paediatric oncology unit.
Cognizant of these factors, the Ethiopian Federal Ministry of Health (FMoH) recently developed a National Childhood and Adolescent Cancer Control Plan (NCACCP) for the years 2019–2023 with the aim of improving survival rates through early detection and diagnosis, quality treatment and supportive care.19 The overall goal is to achieve at least a 40% cure rate for common and curable childhood and adolescent cancers. The timing of the NCACCP plan aligns with the WHO Global Initiative for Childhood Cancer, launched in 2018, which aims to improve survival to at least 60% and to decrease cancer-related suffering for all children with cancer by 2030.22 One means by which the FMoH aims to achieve these targets is by increasing the number of fully equipped and functional paediatric oncology centres in the country from three in 2019 to eight before the end of 2023.19
In general, there is limited evidence on the cost, cost-effectiveness and affordability of paediatric cancer units in LMICs, but a few studies have found that treatment of certain paediatric cancers can be highly cost-effective in such settings. A 2019 systematic review of childhood cancer treatment in LMICs indicates that the cost per DALY averted could range from US dollars (USD) 22 to 4475, which is less than one time the gross domestic product (GDP) per capita of the studied countries, indicating that selected interventions are cost-effective23; the wide range of the result is explained by the difference in cost-component accounting among studies. Similarly, a study conducted in 2021 in four African countries (Kenya, Zambia, Nigeria and Tanzania) found that costs per DALY averted were less than 0.3 times the GDP per capita of Tanzania and Zambia.24 A 2013 study on the cost-effectiveness of acute lymphoblastic leukaemia and Burkitt’s lymphoma treatment in Brazil and Malawi concluded that running a paediatric oncology unit in LMICs would be highly cost-effective by the standard of the WHO-CHOICE cost-effectiveness threshold.25 Other studies conducted at paediatric oncology units in El Salvador and Ghana support these findings, with cost per DALY averted estimates of USD 1624 and USD 1034, respectively,26 27 which is very cost-effective according to the countries’ cost-effectiveness thresholds as determined by the WHO-CHOICE framework.
Despite this promising evidence from other LMICs, a need remains for more country-level evidence because of differing disease burdens, patients’ survival rates, cost of care profiles and willingness to pay (WTP) in Ethiopia compared with other LMICs. Furthermore, local cost-effectiveness evidence could enhance advocacy, trust and policy prioritisation for childhood cancer programmes in the national priority-setting process. As an example, the Ethiopia Essential Health Service Package (EEHSP)28 classifies most childhood cancer diagnostic and treatment services as either low or medium priority despite the aspirational goals of the NCACCP and the recent global attention and advocacy for countries to invest in childhood cancer control; this represents a setback in Ethiopia’s childhood cancer control efforts, which will continue to be underfinanced and out of the leadership’s attention. These priority rankings were partly influenced by a lack of contextualised cost-effectiveness evidence, and the decision was based on experts’ judgement. Therefore, this research aimed to fill the local evidence gap regarding the cost-effectiveness of childhood cancer treatment (specialised paediatric oncology care delivery) to inform the revision of the EEHSP and harmonise the conflicting priority level of childhood cancer treatment between the NCACCP and the EEHSP.
Methods
Study setting
Ethiopia, a country with a population close to 110 million in 2019,29 formerly had only one paediatric oncology unit nationally, located at TASH in Addis Ababa, Ethiopia’s capital. Recently, three additional paediatric oncology centres (in Jimma, Gondar and Mekelle University Hospitals) were added. The costing part of this study was conducted at TASH, which has 81 clinical departments, a 735-bed capacity and close to 500 000 outpatient department (OPD) visits per year in 2019. TASH’s paediatric oncology centre has a capacity of 42 beds, and most suspected cases of childhood cancer (age <15 years) across the country have until recently been referred to this centre. The paediatric oncology unit is financed mainly by the government. The unit has an inpatient department embedded in the main compound of TASH and a satellite clinic proximal to TASH (around 1 km away). The satellite clinic not only serves mainly as an OPD but also provides inpatient services for short admissions to administer chemotherapy. Although the paediatric oncology unit is far from ideally staffed and equipped,7 it has paediatric oncologists, nurses trained in paediatric oncology services, social workers and dedicated pharmacists. Some clinical support services are shared with other departments, such as the laboratory, pharmacy, imaging, pathology, surgery, intensive care unit (ICU), emergency, radiotherapy, blood bank and non-medical central services, such as food, laundry, utilities (eg, electricity and water) and other operational costs.
Decision analytic model
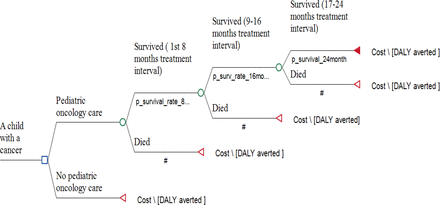
We built a decision analytic model—a decision tree—to estimate the cost-effectiveness of running a paediatric oncology unit compared with a do-nothing scenario from a provider perspective (figure 1). As time and recurrence are important considerations in shaping the natural course of cancer patients, state transition models (a cohort-level or individual-level microsimulation) applied to specific childhood cancer types would have been an ideal approach but that would require very detailed epidemiology and effectiveness data for each cancer type from Ethiopia or at least from similar settings to properly map the various clinical scenarios of patients over time (eg, remission, disease progression, recurrence, death) and justifiably populate the state transition models. Lacking such data, we used a decision analytic model and limited the scope of the study to providing only a gross overview of the cost-effectiveness of paediatric oncology care (at a service-platform level) compared with no paediatric oncology care to inform the national-level policy dialogue. The cancer-specific cost-effectiveness will be incorporated and addressed as more data become available in the future.
A decision-analytic model structure (decision tree) with an average 2-year childhood cancer treatment duration divided into 8-month treatment intervals. The model compares a simulated child with cancer (without a specific diagnosis) who receives services from the paediatric oncology unit to a do-nothing scenario (defined as no paediatric oncology care). The p_survival_rate_8 represents the probability of survival in the first 8 months of treatment. Similarly, p_survival_rate_16 is the probability of survival in 9–16 months of treatment, and p_survival_rate_24 is the probability of survival in 17–24 months of treatment. DALYs, disability-adjusted life years.
We created a generic model simulating a child with cancer (without specifying the diagnosis) who receives services from the paediatric oncology unit (labelled as paediatric oncology care in figure 1) compared with a do-nothing scenario (labelled as no paediatric oncology care). To estimate costs and effects, the model depicts 2 years of treatment (considering an average cancer treatment duration) divided into 8-month treatment intervals. We considered the average treatment duration to be around 2 years, as acute lymphoblastic leukaemia (which can take more than 3 years of treatment) was the dominant type of cancer at TASH, and we took estimates from other centres with comparable cancer patterns.30 31 An 8-month treatment interval was chosen, as the reported median time for events to occur (abandonment or death related to relapse, disease progression, treatment toxicity or background death) is around 8 months.30 31 For the no paediatric oncology care scenario, we assumed that all patients would die at the end of 6 months. For cured children, our model assumes that some survivors will develop late-treatment chronic complications that will affect their quality of life and shorten their life expectancy compared with other children with background mortality. Two outcomes—survival (event-free survival (EFS)) and death (non-survival)—were used to estimate cost and effects at the end of each 8-month treatment interval, and the probabilities for EFS and death were taken from a literature review in similar settings (table 1 and online supplemental text S1 and tables S2 and S3). Abandonment, a significant problem in Ethiopia (around 34%),32 was taken as an event and captured as equivalent to death in our model for the following reasons: (1) most childhood cancer patients in Ethiopia and LICs are diagnosed at a late stage (stage 3–4), and most patients abandon care at an early stage of the treatment phase (due to refusal to start or early discontinuation)21 31 33 34; thus, the chance of survival after abandonment is likely very low35; (2) TASH was the only oncology centre in Addis Ababa, making it unlikely that children would find alternative better treatment elsewhere in the country after abandoning care at the oncology unit unless they travelled abroad; (3) if children accessed treatment in private health facilities (in the country or abroad), the cost would fall on the patients’ guardians and could not be captured in our model, which is from the provider perspective.
Supplemental material
Model parameters, value ranges and type of distribution used in the probabilistic sensitivity analysis (PSA)
The disability of surviving patients was assumed to be better than non-surviving in each treatment interval (table 1). Surviving patients in each treatment interval were assumed to have a better utility compared with their earlier treatment interval status to account for response to treatment and reduced risk of treatment-associated toxicity. Hence, the disability weight progressively fell as they moved from the first 8-month interval to the second (9–16 months), third (17–24 months), and once cured. The disability weight at the first 8 month treatment interval was 0.37, while it was 0.29 at 9–16 months, 0.20 at 17–24 months and 0.07 for cured. The disability weights are taken from the 2019 Institute for Health Metrics and Evaluation estimate for childhood cancer36 and are measured on a scale of 0–1, in which 0 equals perfect health and 1 equals death (table 1).
Model parameter inputs and assumptions
The cost-related model parameters were generated through primary data collection (described below), and the health benefit parameters were taken from a literature review of comparable settings, as no local data were available (table 1 and online supplemental text S1 and tables S2 and S3). We conducted a scoping literature review to identify studies documenting the effectiveness of childhood cancer treatment in African LICs. The literature search was done in six electronic databases, including PubMed, Embase, ScienceDirect, Scopus, Web of Science and African Journals OnLine by combining terminologies covering the spectrum of childhood cancer types, country names (LICs in Africa) and treatment outcomes (survival or mortality). We identified 14 studies fulfilling our criteria and prioritised the evidence based on systematic review or meta-analysis, followed by prospective studies based on cancer registries, multicountry/multicentre studies, and those with large sample sizes, broad cancers coverage, long survival periods and recently conducted studies. We substantiated the survival rate findings from the scoping review using experts’ judgements and local evidence on treatment abandonment and survival rates drawn from expert opinion (online supplemental text S1). We set a modest survival rate in our model to avoid biased cost-effectiveness conclusions. We assumed the 2-year childhood cancer survival rate at TASH to be 25%, with a 95% CI of 15% to 35%, despite commonly reported overall survival rates ranging from 35% to 45% in paediatric oncology centres in LICs in Africa. Further details on the scoping review process, key findings and transferring approach are provided in the online supplemental text S1 and tables S2 and S3.
Estimation of cost
We conducted a costing study (8 July 2018–7 July 2019) to estimate the annual cost of running the paediatric oncology unit at TASH from a provider perspective, using a mixed (top-down and bottom-up) costing approach (for further details, see, Mirutse MK, Palm MT, Tolla MT, Memirie ST, Kefyalew ES, Hailu D, Norheim OF. Cost of childhood cancer treatment in Ethiopia, submitted for publication). We identified, measured and valued the cost inputs used in running the unit. Direct cost inputs—costs directly attributable to a specific department or service output, such as costs of human resources, drugs/supplies and medical equipment—were computed by estimating the amounts consumed by the unit in a year (consumed quantity) multiplied by their unit costs. The costs of shared departments or services—including laboratory, radiation, imaging, pathology, surgical operating room, ICU, paediatric emergency services, inpatient food services, laundry, utilities (rent, electricity, telecommunication, water and other utility charges) and other overhead costs (operating expenses such as office supplies, printing, educational supplies, fuel, per diems and training costs)—were costed by allocating the share of those services used by the paediatric oncology unit; we used various allocation bases appropriate to each case (for further details, see, Mirutse MK, Palm MT, Tolla MT, Memirie ST, Kefyalew ES, Hailu D, Norheim OF. Cost of childhood cancer treatment in Ethiopia, submitted for publication).
Finally, the total cost of the unit was computed by adding the direct cost, the indirect costs from the intermediate departments and the overhead cost. We converted the total cost to USD using the mean exchange rate for 2019. We computed the number of OPD visits per patient during the 8 months, cost per OPD visit, 8-month bed days per patient and cost per bed day. The 8-month OPD visits per patient were computed by dividing the total annual OPD visits of the paediatric oncology unit (7842) by the annual number of patients (1345), and this annual estimate was adjusted for 8 months (taking an 8 month share). The same techniques were used for the 8-month bed days per patient by using the total annual bed days (12 180) and annual number of patients. The costs per OPD visit and per bed day were calculated by integrating the annual OPD and IPD cost estimate and the annual OPD and IPD utilisation statistics report. Then, for each 8-month treatment interval, we estimated the cost of OPD and IPD in each arm and aggregated the total cost. We used the costs of OPD and IPD of non-surviving patients as 1.5 and 2 times the costs of OPD and IPD of surviving patients, respectively, as they are likely to use more and/or expensive services. These estimates were derived from the costing study at TASH, taking into account the cost distribution between regular OPDs and departments related to critical patients and the anticipated service utilisation patterns between surviving and non-surviving patients. However, it is also possible the cost of non-surviving patient to be lower than surviving patient given the high rate of treatment abandonment in Ethiopia, which affects the non-surviving arm in our model and such assumption lowers the cost of running the paediatric oncology unit at TASH (as the model assumes the overall survival rate at TASH to be 25%); hence, it will shift the conclusion towards cost-effective and vice versa in the case of surviving patient cost more than non-surviving patient assumption. We chose a more conservative assumption (the non-surviving patient costing more than surviving patient) so as not to bias the results towards overstating cost-effectiveness and as the alternative assumption will not change the conclusion.
We discounted costs using the global discounting rate (3%)37 for 1 year, as cost was captured only over a 2-year treatment period.
Estimation of health benefits
We used the number of DALYs averted as the effectiveness measurement metric.38 39 The following formula was used to compute the DALYs:

For the no paediatric oncology scenario, we estimated the YLD by assuming that patients would survive for only 6 months without treatment (we multiplied the disability weight without treatment by the average survival duration) (table 1), and we computed the YLL by taking the difference between the age of death and life expectancy at that specific age. We compared both scenarios to a theoretical worst-case situation in which a child dies immediately after cancer diagnosis.
To estimate DALYs averted, we used combinations of model variables (table 1): annual number of new cases, average age at diagnosis, average duration of treatment, EFS rate at end of treatment intervals, life expectancy at specific age, life expectancy gap related to late recurrence or late treatment adverse effects and disability weight. Table 1 gives further details on the model variables, range of values and assumptions. As there is no cancer survival registry or previously conducted childhood cancer health outcome studies in Ethiopia, treatment outcome-related data were taken from evidence in similar settings.4 30–32 40–46 We did not use treatment outcome data from high and middle-income countries, as such outcomes would require further investments in quality improvements that were not captured in our costing estimate. We discounted DALYs averted by 3% using a lifetime horizon to bring future benefits to present value.
Cost-effectiveness analysis
Cost-effectiveness in this generic model was expressed as the incremental cost-effectiveness ratio (ICER) and computed by dividing the incremental costs of introducing a specialised oncology unit by the incremental DALYs averted, that is, due to interventions.

An intervention was considered cost-effective if the ICER was less than 50% of the Ethiopian GDP per capita, and not cost-effective if otherwise.43 We used TreeAge software to build the decision model and run the cost-effectiveness analysis.
Uncertainty
We varied cost, EFS, life expectancy gap after treatment and disability weights using the 95% CI reports from the literature review to estimate the effect of the model variables’ uncertainty on the estimated result (table 1). We conducted a one-way sensitivity analysis and probabilistic sensitivity analysis (PSA) with 100 000 Monte Carlo simulations using various distributions (table 1).
Patient and public involvement
This project did not include patients or the public in developing the research questions or designing and conducting the study. There is a plan to disseminate the results of the study to various stakeholders, including associations and civil societies working on childhood cancer control programmes in Ethiopia.
Results
A total of 1345 children with cancer were treated at TASH from 8 July 2018 to 7 July 2019. The most common cancer types were acute lymphoblastic leukaemia (28%), Wilms tumour (15%) and Hodgkin’s lymphoma (12%), followed by rhabdomyosarcoma, retinoblastoma, neuroblastoma and non-Hodgkin’s lymphoma (further details included in online supplemental table S1). The total cost of a running paediatric oncology unit per treated child (for 2 years) was USD 901, while it was USD 18 for the no paediatric oncology care scenario (6 months). The IC was USD 876 per treated child. The DALYs averted per treated child for an operating paediatric oncology unit were 2.49, whereas the figure was 0.06 for no paediatric oncology care, and the IE per treated child was 2.43. The ICER was USD 361 per DALY averted (table 2).
ICER of running a paediatric oncology unit compared with no paediatric oncology care at TASH in 2019
The tornado diagram (figure 2) presents the variables and range of values tested in the one-way sensitivity analysis. The length of the horizontal bar indicates an individual variable’s potential level of parameter-impact uncertainty on the ICER estimate. The longer the bar, the greater the impact in the direction of the bar (to the left or right). Accordingly, the five parameters with the greatest potential influence on the ICER estimate were cost per bed day, EFS rate in the first 8 months, cost per OPD visit, EFS rate at 17–24 months and life expectancy gap. In the one-way sensitivity analysis, the uncertainty of individual parameters did not alter the cost-effectiveness conclusion, as the level of impact was lower than the WTP threshold for all individual parameters. We varied the cost of the no paediatric oncology scenario down to zero, but it had a minimal effect, slightly increasing the ICER from USD 362 per DALY averted in the base case to USD 370 per DALY averted.
Tornado diagram of the results of the one-way sensitivity analysis of the cost-effectiveness analysis of running a paediatric oncology unit in Ethiopia, summarising the key variables tested for one-way sensitivity analysis, the ranges of values tested and their impacts on the ICER estimate. The longer the horizontal bar, the greater the impact in the direction of the bar (to the left or right). ICER, incremental cost-effectiveness ratio.
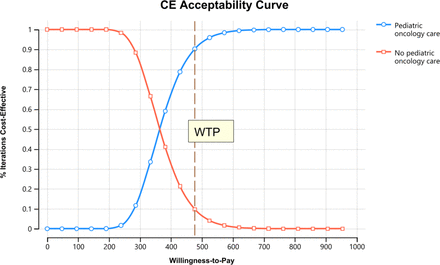
Figure 3 presents the PSA results. At a WTP of <USD 361, the no paediatric oncology care scenario had a higher probability of being cost-effective. At a WTP of USD 361, the two scenarios had an equal probability of being cost-effective (where the red and blue lines cross in figure 3), and the probability of cost-effectiveness was higher for paediatric oncology care at a WTP of >USD 361. The probability of paediatric oncology care being cost-effective was 100% at a WTP of >USD 600.
Probabilistic sensitivity analysis for the cost-effectiveness of running a paediatric oncology in Ethiopia. The figure depicts the range of WTP thresholds in which the no paediatric oncology care scenario will have higher probability of being cost-effective compared with paediatric oncology care (WTP<USD 361), indicates when two scenarios reach equilibrium (WTP=USD 361), and shows when the probability of cost-effectiveness of paediatric oncology care will be higher (WTP>USD 361). WTP, willingness-to-pay.
In our model, running a paediatric oncology unit was cost-effective compared with a no paediatric oncology care scenario in 90% of the Monte Carlo simulations (100 000 simulations) at a WTP of USD 477 (based on 50% of GDP per capita for Ethiopia in 2019) as indicated by the broken brown line in figure 3. The highest ICER estimate from the PSA was around USD 600 per DALY averted.
Discussion
Running a paediatric oncology unit is more effective (2.43 DALYs averted per child treated) than a no paediatric oncology care scenario, but it also costs more (USD 876 per child treated). The ICER of running a paediatric oncology unit compared with the no paediatric oncology care scenario is USD 361 per DALY averted, and it is cost-effective using a USD 477 WTP threshold (50% of Ethiopia’s 2019 GDP per capita), which is a lower threshold than the commonly used WHO-CHOICE-recommended threshold for very cost-effective interventions (lower than the 1 x GDP per capita (USD 953) for Ethiopia).37 47 The results of the Monte Carlo simulation (100 000 iterations) indicate a 90% chance that the ICER will be below the WTP threshold (being cost-effective). As indicated by the one-way sensitivity analysis, the chance of being cost-effective increases with an improvement in survival rate, which is currently very low in Ethiopia.21 The WHO Global Initiative for Childhood Cancer and the Disease Control and Priority Cancer module indicate that investing in childhood cancer control programmes will improve survival and is highly cost-effective, affordable and feasible in LMICs7 22 with prioritisation of certain cancer types, such as acute lymphoblastic leukaemia, Hodgkin’s lymphoma, Burkitt’s lymphoma, retinoblastoma, Wilms tumour and low-grade glioma (brain tumour). Our ICER finding in the generic model is similar to estimates from Tanzania (USD 323 per DALY averted), higher than reports from Uganda (USD 97 per DALY averted)48 and lower than reports from Zimbabwe (USD 537 per DALY averted), Ghana (USD 1034 per DALY averted) and Nigeria (USD 2940 per DALY averted).24 27 The lower ICER estimate in Ethiopia may be related mainly to the low annual cost estimate, which is possibly explained by Ethiopia’s low human resource payment scale, heavily subsidised utility costs (eg, water, electricity), service quality differences, unconsidered cost inputs (explained in the limitations discussion), differences in volume of service provided (the high patient volume in TASH compared with that in the other countries could reduce the cost per treated patient) and differences in treatment protocols, childhood cancer patterns and cost-effectiveness analysis approach.
With an annual cost of USD 577 per treated child (which could be as high as USD 1085 when adjusted for suboptimal care), the budget impact of investing in childhood oncology care may be optimistic, as the population in need of care is small (an annual incidence of childhood cancer of around 3800). Beyond its high potential for cost-effectiveness and low budget impact (hence affordability), investing in paediatric oncology treatment could contribute to reducing financial hardship and improving equity. According to a 2014 WHO report, Making Fair Choices on the Path to Universal Health Coverage, one definition of the worst off is those with the largest individual disease burden, and children with cancer qualify for that definition, as they face high premature death.49 Furthermore, the Ethiopia Health Sector Transformation Plan and Health Equity Strategic Plan place due emphasis on addressing inequity, and children are among the prioritised groups.50 51
In the current EEHSP,28 childhood cancer services are less prioritised; for example, three of the six high-priority childhood cancers identified in the WHO Global Initiative for Childhood Cancers and the Disease Control Priorities—Burkitt’s lymphoma, retinoblastoma and Wilms tumour—are classified as low priority, and two (acute lymphoblastic leukaemia and Hodgkin’s lymphoma)7 22 are classified as medium priority. This may be due to various factors, including a lack of local cost and cost-effectiveness data (leading to a decision based on expert judgement), limitations related to transferring evidence from other countries to Ethiopia’s context and the general perception of a high cost of cancer care and of non-affordability in Ethiopia. Suboptimal engagement and alignment with key stakeholders (within and outside the sector) in the childhood cancer programme may also contribute to this; for example, the goals and target set in the NCACCP contradict the EEHSP revision’s priority results, although both were developed by the same organisation and the EEHSP was endorsed soon after the NCACCP. Our results support recent calls by WHO to emphasise childhood cancer, and they provide evidence for the NCACCP strategy to expand paediatric oncology units in Ethiopia.
Our study has many limitations in terms of cost and effect estimation. The true cost of running a paediatric oncology unit may be larger than our estimate for the following reasons: (1) our estimate did not capture the start-up capital investment, such as building costs and the cost of training specialists (eg, oncologists, specialised nurses and pathologists); (2) the availability of critical diagnostic service, imaging, drugs and supportive care may be suboptimal; (3) direct non-medical costs (eg, transport, lodging) and indirect costs were not captured in our costing exercise; (4) the cost of late treatment adverse effects was not captured; (5) cancers that require advanced and costly diagnosis and treatment such as radiotherapy may not be well represented in our study as such treatment was not readily available in TASH and (6) despite the rigorous data validation conducted, data quality concerns persist in regards to hospital records in general, and it is almost certain that it was not possible to correct all data errors; this may have introduced bias in the form of both overestimation and underestimation of costs, but underestimation is the highly likely case. Since the cost-effectiveness analysis was conducted for a service delivery platform using average costs and average health outcomes, our model does not capture the clinical scenarios a patient might encounter during the treatment period, and the heterogeneity of childhood cancers could present differences in unit costs and health outcomes and, consequently, differences in ICER values. As we lacked a survival registry and previous local health outcome estimates, our model relied on reports from similar settings, which may not be as comparable as assumed. However, we tried to mitigate the limitation by adopting cautious survival values. Furthermore, the potential impact of these limitations on the ICER estimate was explored in the sensitivity analysis, which considered a reasonable range of input parameters and found minimal to no effect on the final conclusions. Around 90% of the ICER iteration results were below the WTP threshold, indicating the relevance of our results. The highest ICER estimate in the PSA is USD 600 per DALY averted, which is fairly close to the WTP.
Conclusions and recommendations
The provision of paediatric cancer services using a specialised oncology unit is most likely cost-effective in Ethiopia, at least for easily treatable cancer types in centres with minimal to moderate capability. Our findings support Ethiopia’s NCACCP strategy to expand childhood oncology units in the country. We recommend reassessing the priority-level decision regarding childhood cancer treatment in the current EEHSP. Childhood cancers’ specific cost-effectiveness estimates, along with budget, financial risk protection and equity impact analysis (which can indicate heterogeneity), could better inform prioritisation among childhood cancers. Improving the childhood cancer information system, including establishing a cancer registry in Ethiopia, is crucial to informing the childhood cancer control programme with robust evidence.
Data availability statement
Data are available upon reasonable request. The data sets used and/or analysed in the current study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
We thank the University of Bergen, the Norwegian Agency for Development Cooperation (NORAD), and the Trond Mohn Foundation for funding our work and the Ethiopia Health Insurance Agency for its leadership and administrative support. We also thank Bjarne Robberstad, Stéphane Verguet, and David Watkins for their valuable comments on an earlier version of the manuscript.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors MK led the entire study (study planning, design, data collection and analysis, results interpretation and manuscript write-up) and he is the guarantor of the study. MTP provided critical inputs in the study design, data collection, analysis and manuscript write-up. OFN, MTT and STM provided supervisory support, reviewed, and provided critical inputs to the study proposal, data collection tools, results, and manuscript. DH provided critical inputs in data collection, results interpretation, and enriching of the manuscript. All the coauthors reviewed and endorsed the final manuscript.
Funding This work was supported by the University of Bergen, the Trond Mohn Foundation (grant no. BFS2019TMT02), and the Norwegian Agency for Development Cooperation (NORAD) (grant number RAF-18/0009) through the Bergen Centre for Ethics and Priority Setting (BCEPS: project number 813 596).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.