Article Text
Abstract
Objective Dry eye disease (DED) is a multifactorial disease involving the tears and ocular surface. It impacts a patient’s quality of life (QoL) and ability to perform daily activities. This study assessed the burden of self-reported DED among adults in eight European countries.
Design Online cross-sectional survey.
Setting General population in France, Italy, Germany, Greece, the Netherlands, Portugal, Spain and Sweden.
Participants Adults aged ≥18 years with (n=6084) and without (n=6161) self-reported DED were recruited via emails and screened.
Main outcome measures All participants completed National Eye Institute Visual Function Questionnaire-25 (NEI-VFQ-25) and EuroQol-5 Dimension-5 Level Questionnaire (EQ-5D-5L). All DED participants completed the Eye Dryness Score (EDS) Visual Analogue Scale, and Ocular Comfort Index and Work Productivity and Activity Impairment Questionnaire: Specific Health Problem questionnaires. In addition, half of the respondents with DED completed Survey A (Impact of Dry Eye on Everyday Life) and the other half completed Survey B (Standard Patient Evaluation of Eye Dryness Questionnaire) and Dry Eye Questionnaire-5.
Results Participants with self-reported DED had lower functional vision and lower overall health status than participants without self-reported DED as measured by the NEI-VFQ and EQ-5D-5L, respectively.
Increasing self-reported DED severity as measured by the EDS was shown to correspond with worse symptom severity/frequency, lower functional vision, higher impact on work productivity, daily activities and QoL.
Conclusion This study showed that patients’ reported burden of self-reported DED was similar across the eight European countries. Those with self-reported DED reported lower health status and functional vision compared to those without self-reported DED and these parameters worsen with increasing disease severity.
- ophthalmology
- epidemiology
- primary care
- corneal and external diseases
- public health
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. We confirm that the data generated by our research supports our current article. We confirm that we have included all our generated data in this manuscript. Novartis is committed to sharing with qualified external researchers, access to patient level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymised to respect the privacy of patients who have participated in the online surveys in line with applicable laws and regulations. The authors confirm they had no special access or privileges that other researchers would not have. Unfortunately, we are unable to provide copies of the survey as the questions asked were from copyrighted questionnaires.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This comprehensive study is the largest Dry Eye Disease (DED) survey completed in Europe including eight different countries assessing multiple health-related and vision-related quality of life aspects among participants with and without self-reported DED, which may be considered as a strength of the study.
To our knowledge, this is the first study to assess the impact of self-reported DED on work productivity and Healthcare Resource Utilisation in Europe.
Considering this study was an internet-based survey, individuals without access to the internet, and individuals who were not computer literate, especially the elderly who would have a higher prevalence of self-reported DED, may not have had to opportunity to participate in the study.
DED participants were recruited through self-reported diagnosis of DED or by having two or more of the typical symptoms for DED, as such diagnosis of DED was not confirmed. This may have resulted in inclusion of participants who were unaware that they have DED and were not seeking treatment or visiting a healthcare professional.
Patients with severe DED might have required help from another individual to complete the survey, which may have led them to decline to participate; participants with DED may also decline to participate in surveys where they would have to use a digital screen.
Introduction
Dry eye disease (DED) is a common condition for which affected individuals regularly seek medical attention.1 2 The prevalence of DED is high with a variable reported range. Worldwide estimates in adult populations range from 5% to 50%,2 with European estimates ranging between 10% and 30%.3–6 Ambiguity in diagnostic criteria contribute to the uncertainty in decision making.7 Clinical manifestations of DED often poorly correlate with known characteristic signs and symptoms of the condition.8 Thus, manifestation of DED is often underestimated or misdiagnosed by caregivers, physicians, institutions, and society in general and diagnoses may be delayed.9
DED has a multifactorial aetiology involving the entire ocular surface.10–13 The key pathophysiology elements of DED are abnormalities of tears (short break-up time) and of ocular surface tissues (corneal and/or conjunctival epitheliopathy). The aetiological mechanisms involved in DED are very diverse, resulting in a self-sustained condition that eventually affects all partners of the lacrimal function unit (including corneal nerves, meibomian glands in the eyelids, lacrimal glands and even emotional centres associated with chronic pain perception). This vicious cycle of events on the ocular surface leads to symptoms of discomfort and visual impairment with a significant impact on visual tasks. Ocular symptoms vary widely ranging from minor transient irritation, to continuous itchiness, burning, dryness, pain, redness, visual disturbances and ocular fatigue.10 Daily activities including driving, reading and more generally any use of electronic screens (computers, television, phones) may be negatively affected, as well as performance and productivity in the workplace, with higher rates of both absenteeism and presenteeism reported.14–16 Additionally, DED is a cause of chronic pain, which explains the physical fatigue and the substantial effects on mental health (psychological stress, sleep disorders, anxiety, depression) that have been reported.2 17 18 Overall, there is a significant impact of DED on quality of life (QoL) and functionality.11
Several studies have shown that DED is associated with a substantial economic burden due to direct (eg, specialist consultation, medication and surgical costs) and indirect costs (eg, absenteeism and presenteeism) as highlighted in the Tear Film and Ocular Surface Society Dry Eye Workshop (DEWS) II Epidemiology Report.2
A systematic literature review confirmed that the largest proportion of costs are attributed to indirect costs due to reduced productivity at work.19 However, no literature was identified reporting on DED-related productivity loss or indirect costs in Europe.
We report the results of a patient survey, which aimed to assess the burden of self-reported DED among adults in eight European countries. The objective of the present study was to assess country-specific descriptive data in a large number of subjects; however, the study was not designed to compare the burden of DED between countries.
Methods
Study design
A cross-sectional web-based study was conducted between February and May 2019, in adults aged ≥18 years, across the eight European countries—France, Italy, Germany, Greece, the Netherlands, Portugal, Spain and Sweden.
The study was advertised via email to individuals who had previously elected to receive invitations for ethics-approved healthcare-related studies. Participants answered study eligibility questions via a web-based screener and consented electronically before beginning the survey. The online survey was developed using Confirmit Horizon, which is a web-based platform. Respondents received a nominal compensation for their time after completion of the survey from the marketing agency which recruited them. The costs were reimbursed by the study sponsor. Participants were included in a panel, where they could earn ‘panel points’ for participation and receive compensation with these points. The number of panel points varied depending on the duration of participant’s tenure in the panel and were in line with local regulations/guidelines. All data were anonymised. The study documents, including screener, consent form and study questions, were approved by a central institutional review board.
Two convenience samples were recruited: (1) participants with self-reported DED and (2) participants without self-reported DED. Participants were asked to select any medical condition they experience from a list of conditions (online supplemental table 1). Those who indicated a diagnosis of DED from the list of medical conditions were included in the DED group. Participants were also included in the DED group if they reported having at least two of the six most common symptoms of DED at the time of the survey (eye discomfort, including feelings of dryness, grittiness or soreness in the eyes; burning sensation in the eyes; feeling as if something is in the eye; eyelids that stick together on waking; temporarily blurred vision, which usually improves blinking; tired eyes).
Supplemental material
All participants completed the National Eye Institute Visual Functioning Questionnaire-25 (NEI-VFQ-25) and the EuroQol-5 Dimension-5 Level Questionnaire (EQ-5D-5L). Outcome variables assessed for DED participants included symptoms, impact of the disease, work productivity and health-related QoL (HRQoL) measures, related to ocular discomfort, functional vision and health status (online supplemental table 2). Respondents with DED completed the Eye Dryness Score (EDS), a Visual Analogue Scale (VAS) assessing the level of discomfort in the eyes with regard to the symptom of eye dryness in the past 24 hours, ranging from 0 (no discomfort due to eye dryness) to 100 (maximal discomfort due to eye dryness) developed in conjunction with clinical studies for patients with DED. In DED clinical studies, eye dryness was one of the most common and severe symptoms reported at baseline and the EDS (VAS) appeared to be a reliable and sensitive measure.20 21 All participants in the self-reported DED sample completed the Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI) and the Ocular Comfort Index (OCI). Given that the existing literature provided minimal evidence to justify only one specific symptom and impact instrument for DED, it was decided to include the most frequently used instruments. As several questionnaires were to be completed, to reduce completion burden, half of the self-reported DED cohort completed the Impact of Dry Eye on Everyday Life (IDEEL) questionnaire, referred to as ‘Survey A,’ and the remaining participants with DED completed ‘Survey B,’ which comprised the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire and the Dry Eye Questionnaire (DEQ-5) (online supplemental table 2). Allocation to survey A or survey B was through random assignment. Survey A and survey B, each took approximately 45 min for completion. Participants could take a break during the survey and on reentry, could continue from where they left off. Participants who completed the screener but did not have DED were asked to complete a 20 min survey that assessed general demographic characteristics and QoL.
Participants with self-reported DED were surveyed about direct medical requirements of managing DED in the 12-month period prior to taking the survey. This included the frequency and type of healthcare professional (HCP) visited (ie, ocular specialist (eg, ophthalmologist, optometrist) or non-specialist (general practitioner, nurse)). DED-related out of pocket (OOP) expenses were reported for healthcare visits, pharmacological treatments and medical interventions requiring hospitalisation or surgery. Participants also reported the amount of time they had been absent from work in the past 7 days because of their self-reported DED.
Recruitment targets for the countries with a large population such as France, Germany, Italy and Spain were 1000 participants with self-reported DED and 1000 without self-reported DED. In Greece, Portugal, Sweden and the Netherlands, countries with smaller populations, recruitment targets were 500 participants with DED and 500 without DED.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this study.
Statistical analysis
Sociodemographic, diagnosis, medical history characteristics, visual function and health status (NEI VFQ-25 and EQ-5D-5L) were described for all participants. EQ-5D-5L values were calculated using the country-specific reference set when they were available (Germany, Spain and the Netherlands) or the UK reference set (France, Greece, Italy, Portugal and Sweden). Scores for the VFQ-25 were calculated using the 28-item revised VFQ (VFQ-28-R) scoring.22 To note that the OCI scores were not calculated as per the scoring of the developer (only a sum was calculated, not the Rasch-based scores).
To assess the impact of self-reported DED severity on the burden of disease, three DED severity groups were defined: EDS<40, 40≤EDS<60 and EDS≥60, as defined during clinical trials with DED patients.20 21 23 Scores for each of the patient-reported outcome measures (PROMs) were categorised by EDS severity.
Missing data and/or missing assessments were not imputed. For the calculation of scores, missing data handling was performed according to the specific guidelines of the respective instruments. Data analysis was performed using Statistical Analysis Software V.9.4.
Results
Participant characteristics
A total of 12 245 participants were included in the study from the eight participating European countries: 6084 in the self-reported DED cohort and 6161 in the non-DED cohort. Mean±SD age of participants included across the European countries ranged from 50.6±15.9 years (Greece) to 54.7±15.6 years (Sweden) (table 1).
Baseline and demographic characteristics of the participants included in the study
Respondents in the self-reported DED group reported higher rates of cataracts than the non-DED groups. Cataract was the most commonly reported eye-related medical condition in the self-reported DED group for all countries (15%). Greece had the lowest proportion of participants in the DED group with cataracts (7.2%), while Spain had the highest (18.2%). Other commonly reported eye-related medical conditions in the DED group included glaucoma (6.4%) which ranged from 2.3% in Greece to 10.9% in France (online supplemental table 3). DED participants were asked if they had experienced seasonal allergies. Portugal and Spain had the highest percentage of participants with self-reported DED experiencing seasonal allergies with 53% and 42.2%, respectively. For the remaining countries, proportion of participants reporting seasonal allergies ranged from 32.6% (Germany) to 39.9% (Sweden).
As environmental factors are known to be significant risk factors for DED, all participants were asked about their exposure to certain environmental conditions. The most commonly reported environmental factors reported by a majority of participants included daily use of digital screens, reading and driving in both self-reported DED and non-DED subjects. Participants with DED reported higher exposure to air-conditioned/recirculated air for long durations, low humidity, polluted air and exposing the eye to forced air/heat, wind/moving air (online supplemental table 4).
The proportion of DED participants whose DED was diagnosed by an HCP highly varied between countries: Sweden: 27.9%; Greece: 55.0%; Portugal: 55.9%; the Netherlands: 59.3%; France: 66.4%; Spain: 67.3; Italy: 73.7%; Germany: 76.8%. The mean time since DED diagnosis ranged from 4.4 years in Greece to 7.5 years in Germany.
Health status and functional vision for participants with and without DED
DED negatively affects patients’ general health and functional vision as demonstrated by QoL instruments compared with non-DED subjects.
Results from the EQ-5D-5L, a general health questionnaire, showed lower scores for participants with self-reported DED than those without DED, indicating a lower overall health status. When aggregating across the eight countries, the domains with the highest proportion of participants most affected by DED were pain/discomfort (impacted: 74.5%; extreme and severely impacted: 8.0%), followed by anxiety/depression (impacted: 54.3%; extreme and severely impacted: 6.9%), usual activities (impacted: 31.2%; extreme and severely impacted: 2.6%), mobility (impacted: 29.4%; extreme and severely impacted: 4.2%) and self-care (impacted: 11.9%; extreme and severely impacted: 1.2%) (figure 1).
Description of EQ-5D-5L item responses by self-reported DED status in all participants. Anx., anxiety; DED, dry eye disease; EQ-5D-5L, EuroQoL-5 Dimensions-5 Levels Questionnaire.
When comparing at a country level, the difference between self-reported DED and non-DED participants, who indicated that the pain/discomfort domain was most impactful, was most pronounced in France (84% vs 50%) and least pronounced in Greece (55% vs 39%). In all countries, the self-reported DED group reported higher levels of depression than those without DED. Anxiety/depression was reported as having a negative impact by a large number of subjects in Greece in both self-reported DED and non-DED participants (70% and 66%, respectively) and France (59% and 40%, respectively) (online supplemental figure 1).
DED was associated with a negative impact on socioemotional and functional vision (as assessed by the VFQ-28-R). Participants with self-reported DED had lower VFQ-28-R scores as compared with non-DED subjects in all eight countries. This negative impact was consistent across all VFQ-28-R domains: activity limitation score, socioemotional functioning score and total score (figure 2).
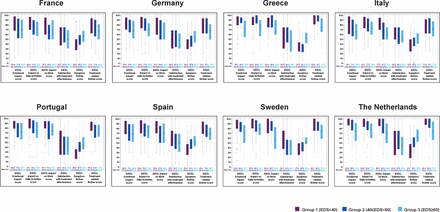
VFQ-28R scores in participants with self-reported DED versus participants without self-reported DED. Box for each score: IQR (Q1–Q3); +: mean; —: median; bottom and top bars: observed minimum and maximum values; ×: outliers (values that are outside the distance of 1.5 times the IQR from Q1/Q3). Score range: 0–100 (lower score indicates more impact on visual function). DED, dry eye disease; VFQ-28R, 28-item revised Visual Function Questionnaire.
Impact of DED on functional vision and health status and eye discomfort by severity
Of the participants with self-reported DED (n=6084), about half were in the mildest severity group (EDS<40), ranging from 41.5% in Italy, 57.7% in Portugal, 64.5% in Greece, with other countries having proportion between 46.6% and 50.4%. The proportion of patients with most severe self-reported DED (EDS≥60) was lowest in Greece (21.9%) and Portugal (22.9%) and between 28.7% and 33.6% in other countries (table 1).
Aggregate EQ-5D-5L scores for the eight countries for EDS<40, 40≤EDS<60 and EDS≥60 severity groups were: 0.80±0.18, 0.77±0.20 and 0.72±0.23, respectively. Participants with EDS≥60 in France (0.65±0.23) and Sweden (0.64±0.24) were more severely impacted by DED compared with participants in Germany (0.79±0.18), Greece, (0.72±0.21), Italy, (0.69±0.20), Portugal (0.74±0.23), Spain, (0.77±0.23) and the Netherlands, (0.68±0.25) (online supplemental figure 2).
VFQ-28-R scores decreased with increasing EDS severity across the domains of activity limitations, socioemotional functioning and total score. Mean±SD VFQ-28-R total scores for EDS<40, 40≤EDS<60, EDS≥60 severity groups for all countries were 72.2±12.1, 68.7±11.2 and 66.6±11.5, respectively.
VFQ-28-R scores across all three domains showed a greater impact on visual function among participants as self-reported DED severity increased. Highest impact with lowest VFQ-28-R total scores for participants with EDS≥60 was observed in the Netherlands (64.6±10.9), Spain (65.7±11.8), Italy (65.5±10.3) and Germany (67.2±10.8). Highest VFQ-28-R total score (lowest impact on visual function) was observed in Portugal (73.3±11.9) and Greece (75±12.1) in participants with EDS<40 compared with Germany (70.9±11.9), Netherlands (71.8±12.8) and Spain (71.4±11.7) (figure 3). Activity limitations were more affected for all severity groups than socio-emotional functioning especially in participants with EDS≥60 (figure 3).
VFQ-28-R scores by EDS severity groups. Box for each score: IQR (Q1–Q3); +: mean; —: median; bottom and top bars: observed minimum and maximum values; ×: outliers (values that are outside the distance of 1.5 times the IQR from Q1 to Q3). Score range: 0–100 (lower score indicates more impact on visual function). DED, dry eye disease; EDS, Eye Dryness Score; VFQ-28R, 28-item revised Visual Function Questionnaire.
Using the VFQ-28-R, it was shown that impact on activity limitations increased with self-reported DED severity. Mean±SD scores for all countries for patients with EDS<40, 40≤EDS<60 and EDS≥60 were 73.2±12, 70.1±11.3 and 68.0±11.5, respectively. Lowest scores (highest impact) were reported in patients with EDS≥60 in Germany (68.2±10.9), Spain (66.6±11.5) and the Netherlands (66.3±11.4). Highest scores (least impact) were reported by participants with EDS<40 in Greece (75.0±12.0), Portugal (74.5±12.0) and France (74.5±12.2) (figure 3).
Impact on daily activities was also captured in patients with self-reported DED who completed the IDEEL questionnaire (n=3055). Highest impact (lowest scores) was reported in Germany (68.8±19.2), the Netherlands (73.4±22.3) and Italy (72.1±18.2) for patients with EDS≥60. Least impact (highest scores) for the EDS<40 and 40≤EDS<60 categories were reported in Greece with respective scores of 86.5±15.6 and 89.2±9.8 (figure 4). Mean aggregated scores for all eight countries for EDS<40, ≤EDS<60 and EDS≥60 were 84.1±16.4, 78.4±18.0 and 72.3±19.5, respectively.
IDEEL scores by EDS severity groups. Box for each score: IQR (Q1-Q3); +: mean; —: median; bottom and top bars: observed minimum and maximum values; ×: outliers (values that are outside the distance of 1.5 times the IQR from Q1 to Q3). Score range: 0–100 (lower score indicates more impact on functional vision). EDS, Eye Dryness Score; IDEEL, Impact of Dry Eye on Everyday Life.
Similar trends were observed for emotional impact as measured in the VFQ-28-R and IDEEL questionnaires. Germany (68.3±21.3), Sweden (67.7±22.2) and Italy (65±20.9) had the lowest scores in the IDEEL emotional impact domain (figure 4). Lowest VFQ-28-R socioemotional scores for EDS≥60 category were observed in France (67.3±18.8), Italy (65.8±17.6), Spain (70.5±20.5) and the Netherlands (66.4±17.7) (figure 3). In other countries such as Greece and Portugal, there was a lower impact of DED on IDEEL emotional impact scores and VFQ-28-R socioemotional scores observed for patients with EDS<40, in particular, patients in the EDS≥60 category were more impacted; however, there was a relatively small sample of patients under this category in these countries (figures 3 and 4).
The IDEEL questionnaire showed low scores for treatment satisfaction across all severities, highlighting the need for adequate DED treatments (figure 4).
Using a series of DED-specific PROMs such as OCI, SPEED and DEQ-5 confirmed similar trends, as the frequency, severity and intensity of DED symptoms was shown to increase with EDS severity. Mean±SD OCI scores for EDS<40, 40≤EDS<60, EDS≥60 were 24.8±12.6, 32.8±12.3 and 39.2±12.5, respectively. Highest scores were recorded in the Netherlands, France and Germany for OCI, SPEED and DEQ-5 indicating greater frequency, severity,and intensity of DED symptoms. Lower OCI scores for EDS<40 were observed in Greece and Portugal, with Greece and Sweden having the lowest DEQ-5 and SPEED scores, respectively, for this category. This indicated that patients in the EDS<40 group in Greece had quite mild DED symptoms. However, patients with EDS≥60 in Greece had OCI scores that were higher than the average for all countries (41.6±12.1 vs 39.6±12.5) (online supplemental table 5).
Impact of self-reported DED on work productivity
Higher self-reported DED severity, as assessed by EDS, was associated with a higher level of work productivity impairment, especially among participants with EDS≥60. Mean±SD WPAI scores for all countries for percent overall work impairment in EDS 40, 40≤EDS<60 and EDS≥60 (representative of mild, moderate and severe severity groups) were 20.4±23.8, 29.0±27.0 and 31.7±28.2, respectively. Participants in Spain, France, Portugal and the Netherlands reported the highest burden particularly participants with EDS≥60. Notably, participants with 40≤EDS<60 in Portugal had higher percentage impairment while working (29.1±25.2) and per cent overall work impairment scores (31.4±27.5) compared with the mean for all countries for the respective domains (25.8±24.0 and 29.0±27.0) (online supplemental figure 3). Calculations for Italy were not considered in this analysis due to an error in the translation of the instrument, which may have led to participants misunderstanding the question.
Based on answers to specific questions related to work, the mean±SD hours of work missed in the past 7 days prior to the survey (patients with self-reported DED, all severities (n=6084)) was 0.85±4.46, which amounts to over 40 hours (5 workdays) a year (based on 47 weeks of work per year and 8 hours per day). Notably, the majority of participants (88.5% (n=5384)]) stated that they did not miss any time from work during the past 7 days.
Healthcare resource utilisation
DED participant-reported information on healthcare resource utilisation (HCRU) showed an average of 2.76±5.08 DED-related visits to HCPs 1.66±2.96 of which were to an ocular specialist per year. For patients with most severe self-reported DED (EDS≥60), this amounted to an average of 3.65±6.89 HCP visits and 2.21±4.34oc ular specialist visits annually.
A total of 102 (1.68%) patients had a self-reported DED-related hospitalisation and 53 (0.87%) required surgery for their DED. The most commonly used treatments for DED were artificial tears (64.38% (n=3917)) and ophthalmic gels (29.40% (n=1789)).
Mean±SD OOP expenditure for self-reported DED-related appointments, treatments and interventions in the past 12 months was €127.06±€302.88. Mean±SD OOP expenses for EDS<40, 40≤EDS<60 and EDS≥60 were €96.73±€275.71, €139.20±€286.89 and €168.05±€347.18, respectively. Italy had the highest mean OOP of €239.28±€341.13. France had the lowest mean OOP expenditure of €38.02±€188.02. This refers only to costs directly paid by patients with self-reported DED, costs that are mostly not considered when assessing the economic burden of the disease (table 2).
DED-related healthcare resource utilisation per country in the past 12 months prior to the survey
Discussion
This comprehensive study is the largest DED survey completed in Europe including eight different countries assessing multiple health-related and vision-related QoL aspects among participants with and without self-reported DED, which may be considered as a strength of the study. Mean age of the participants across the eight countries was comparable and ranged from 50 to 55 years. Majority of the participants in both self-reported DED and non-DED groups included in this study reported use of digital screens and reading/driving as part of their typical daily environment, although no information was collected about the frequency or duration of screen use. Increased screen time has been associated with increased risk of Computer Vision Syndrome (CVS), which in turn is associated with increased risk of DED. A recent study from Spain showed that participants increased their screen time and computer use during COVID-19 lockdowns. Participants who spent more time on electronic devices and less time outdoors reported more CVS-related symptoms. The burden of DED may increase in the future, especially in the younger population, as a consequence of increased screen time. In addition, our study found that about 33%–53% of participants experienced seasonal allergies. Ocular allergy is a known risk factor for DED, as it may affect different mechanisms of DED, including tear film instability, ocular surface inflammation and damage, and neurosensory abnormalities.24
The impact of DED on functional vision and health status was shown through the VFQ-28-R and EQ-5D-5L scores, with participants with DED reporting more difficulty with vision-related activities and lower health status than participants without DED. VFQ-28-R scores revealed a higher impact, especially for participants with more severe self-reported DED. Li et al, in a comparative, observational study, reported that DED had a negative impact on general health, general vision, ocular pain, short distance vision activities, long distance vision activities, vision-related social function, mental health, role difficulties, vision-related dependency and driving on the NEI-VFQ scale.25
In our survey, increasing EDS severity was associated with worse outcomes in terms of self-reported DED symptom severity and intensity, ocular discomfort, functional vision, health status, work productivity and HCRU. VFQ-28-R scores worsened with increasing severity of self-reported DED as measured by the EDS. Similarly, the OCI, EQ-5D-5L and WPAI showed that DED had a greater impact on vision with increasing severity. The impact of self-reported DED on most domains of the IDEEL questionnaire also showed an increase with the severity of the disease. Both Hossain et al and Dana et al, who presented the results of the same study design performed in the UK and in USA, respectively, also concluded that the burden of self-reported DED was substantial, and that the impact of DED on work productivity and HRQoL increased with disease severity.
There were no analyses conducted to assess correlations between the instruments. The literature review performed prior to the survey showed minimal evidence to justify the choice of only one specific symptom and impact instrument. Therefore, it was decided to include all of them, with this specific study design (to respond to only survey A or survey B) to reduce participant burden. Although no analysis of correlations was performed, the results showed that scores obtained with the different questionnaires led to the same conclusions, which is in line with previous reports.26–28
Recently, a study has shown that the patients’ experience and impact of DED may vary between countries. In 2016, a cross-sectional survey of DED patients was conducted across five European countries, including France, Germany, Italy, Spain and the UK. Overall, 31% of the selected patients perceived DED as a ‘handicap’ or a ‘disease’, and these negative perceptions, as well as delays in diagnosis and frequent use of treatments, were correlated with greater reduction in QoL.29 The consequence of this variability in patients’ experience, and perception of DED suggested that the burden of the disease may possibly vary between countries.
Although our present survey was not designed to compare the burden of DED between countries, the results show that the burden of self-reported DED reported by patients was similar across the eight European countries. Variability between countries appears to be related to the disease severity, for example, milder DED patients in Greece and Portugal (low self-reported DED severity as well as high rate of no-eye-related medical history/procedures) as compared with the other countries. In addition, the results are similar to those obtained for the UK and the USA, who had used the same survey and study design.30 31
However, the survey showed differences between countries in relation to country-specific management of DED and differences in healthcare systems, as captured when assessing the percentage of participants reporting that they were diagnosed for DED by an HCP and/or eye specialist, ranging from 27.9% in Sweden up to 76.8% in Germany. The publication of the collective work of the DEWS II could help with standardisation of signs and symptoms of DED and the management of DED reducing variability between countries.2
To our knowledge, this is the first study to assess the impact of self-reported DED on work productivity in a larger scale involving multiethnic population from Europe. All domains of the WPAI questionnaire were impacted, increasing with self-reported DED severity. While absence from work ranged between 3.3% (mildest self-reported DED) and 9.8% (most severe self-reported DED), in terms of percent work time missed in the past 7 days, this might represent half a day lost per week for severe DED patients. Based on patients’ direct information an average of 0.85±4.46 hours of work were missed in the past 7 days. However, it should be noted that self-reported DED patients may be more likely to report presenteeism, where employees are present at work and their work is impacted due to their condition, rather than absenteeism where employees miss work due to their condition. A meta-analysis from Sivakumar et al showed that Sjogren’s and non-Sjogren’s DED patients experience significant absenteeism, presenteeism, productivity and activity impairment.32
An economic assessment of DED in Europe was performed by Clegg et al. The annual total cost for 1000 patients with DED in Europe managed by ophthalmologists ranged from US$0.27 million in France to US$1.10 million in the UK.33 This analysis did not take into consideration the OOP expenses paid by patients, or the indirect costs, underestimating the true societal costs of self-reported DED borne by both patient and government. Our study gathered evidence on OOP expenses paid by patients, which varied largely by EDS severity with mean±SD OOP expenditure of €127.06±€302.88 in the past 12 months. Patients with EDS>60 reported mean±SD expenses of €168.05±€347.18 (table 2).
Participants in the EDS<40 category, particularly in Greece and Portugal, reported that the burden of self-reported DED was quite low. This may be due to a large proportion of this group having very mild self-reported DED, as patients in this category reported lower OCI, DEQ-5, VFQ-28-R, SPEED and IDEEL scores. This might suggest that more granularity in the lower scoring could be beneficial to help distinguish between patients with self-reported DED having mild symptoms, as opposed to those who do not have DED. Alternatively, this could reflect the well-known discrepancy between signs and symptoms of self-reported DED, and/or reflect the effect of DED treatment in lowering the symptom severity.
Strength and limitations of the study
This comprehensive study is the largest DED survey completed in Europe including eight different countries assessing multiple health-related and vision-related QoL aspects among participants with and without self-reported DED, which may be considered as a strength of the study.
To our knowledge, this is the first study to assess the impact of self-reported DED on work productivity and HCRU in multiethnic population from Europe. This study had several limitations. Considering this study was an internet-based survey, individuals without access to the internet, and individuals who were not computer literate, especially the elderly who would have a higher prevalence of self-reported DED, may not have had to opportunity to participate in the study. DED participants were recruited through self-reported diagnosis of DED or by having two or more of the typical symptoms for DED, as such diagnosis of DED was not confirmed. This may have resulted in inclusion of participants (in non-DED group) who were unaware that they have DED and were not seeking treatment or visiting an HCP, or who had DED of mild severity. Patients with severe DED might have required help from another individual to complete the survey, which may have led them to decline to participate; participants with DED may also decline to participate in surveys where they would have to use a digital screen. The diagnosis of DED by the HCP was one of the questions included in the survey, but was left to the discretion of the patient to report, without proper control.
Considering that this study is the largest cross-sectional online survey conducted in Europe across eight countries, the self-reported DED can be used to further explore country-specific burden of DED. In addition, the country-specific description of DED symptoms and their impact on QOL based on the risk factors may help in the development of future diagnostic tools.
Conclusions
Results of the present survey demonstrate that self-reported DED constitutes a significant burden on patients, specifically on their functional vision and overall health status. DED has a significant impact on patients’ daily activities and work productivity, highlighting the broader socioeconomic impact of self-reported DED. The study highlighted that the impact of self-reported DED, as evaluated by several commonly used PROMs for DED such as the DEQ-5, OCI, IDEEL and SPEED questionnaires, increased with self-reported DED severity. We can see from this study the burden and impact of self-reported DED on QoL across eight different European countries, and the impact by severity levels as measured by EDS. Patients with EDS>60 reported the highest burden across all countries.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. We confirm that the data generated by our research supports our current article. We confirm that we have included all our generated data in this manuscript. Novartis is committed to sharing with qualified external researchers, access to patient level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymised to respect the privacy of patients who have participated in the online surveys in line with applicable laws and regulations. The authors confirm they had no special access or privileges that other researchers would not have. Unfortunately, we are unable to provide copies of the survey as the questions asked were from copyrighted questionnaires.
Ethics statements
Patient consent for publication
Ethics approval
This study was approved by Freiburg Ethics Commission International (code: 017/1868). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors would like to thank Jessica Commane, Liam Marshall, Phillip Cooney (Novartis Ireland, Dublin, Ireland), Sameena Haque (Novartis Pharmaceuticals UK, London, UK) and Charis Lau (Novartis Pharmaceuticals Corporation, USA) for their contribution in the interpretation of data, and Deepshikha Roy and Gurpreet Kaur Virya (Novartis Healthcare, Hyderabad, India) for their medical writing support and editorial assistance.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors KGB, EMM, JB-d-C, JM, BS, PO'B, MJQ, MR and ML contributed to the concept and design of the study. JM performed data acquisition. JM, PO'B and BS collaborated on the analysis and interpretation of data. KGB, EMM, JB-d-C, JM, BS, PO'B, MJQ, MR and ML drafted the manuscript and revised it critically for important intellectual content. PO'B and BS supervised the study. ML is responsible for the overall content as the guarantor. All authors have read and approved the final version of the manuscript.
Funding This study was funded by Novartis Pharma AG, Basel, Switzerland.
Competing interests KGB received honoraria and research grants from Thea, Alcon, Novartis, Bausch Health, Santen. EMM is a consultant or speaker at: Alcon, Chiesi, DMG, Dompé, GSK, Kala, Novartis, Pharm-Allergan GmbH, Santen GmbH, Shire, Sun, Sifi, Théa Pharma GmbH, TRB-Chemedica AG, Ursapharm, Visufarma. JB-d-C is a consultant or speaker at Alcon, Angelini, Esteve, GSK, Lumenis, Novartis, Allergan, Santen, Sifi, Théa Pharma, Visufarma. JM is an employee of Modus Outcomes, and a consultant for Shire, a Takeda company, and for Novartis on this study. BS is an employee of Novartis Pharma AG. PO'B is an employee of Novartis Ireland, Dublin, Ireland. MJQ is a consultant and speaker at, Allergan, Bausch & Lomb, Alimera, Santen, Théa Pharma. MR is a consultant, speaker or research grant receiver at Alcon, Alfa-Intes, Allergan, Baif-International, Bausch & Lomb, Bruschettini, Kala, Medivis, NTC, Off-Italia, Novartis, Pfizer, Santen, Sifi, Sooft-Fidia, Sun Pharma, Takeda, Théa Pharma. ML has acted as a consultant for Alcon, Allergan, Bausch & Lomb, DMG, Dompe, Horus, MSD, Novartis, Quantel, Santen/Novagal, Shire, Sifi, Théa and Topivert.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.