Article Text
Abstract
Objective To estimate prevalence of HIV infection in Nigeria and to examine variations by geopolitical zones and study characteristics to inform policy, practice and research.
Methods We conducted a comprehensive search of bibliographic databases including PubMed, CINAHL, PsycINFO, Global Health, Academic Search Elite and Allied and Complementary Medicine Database (AMED) and grey sources for studies published between 1 January 2008 and 31 December 2019. Studies reporting prevalence estimates of HIV among pregnant women in Nigeria using a diagnostic test were included. Primary outcome was proportion (%) of pregnant women living with HIV infection. A review protocol was developed and registered (PROSPERO 2019 CRD42019107037).
Results Twenty-three studies involving 72 728 pregnant women were included. Ten studies were of high quality and the remaining were of moderate quality. Twenty-one studies used two or more diagnostic tests to identify women living with HIV. Overall pooled prevalence of HIV among pregnant women was 7.22% (95% CI 5.64 to 9.21). Studies showed high degree of heterogeneity (I2=97.2%) and evidence of publication bias (p=0.728). Pooled prevalence for most individual geopolitical zones showed substantial variations compared with overall prevalence. North-Central (6.84%, 95% CI 4.73 to 9.79) and South-West zones (6.27%, 95% CI 4.75 to 8.24) had lower prevalence whereas South-East zone (17.04%, 95% CI 9.01 to 29.86) had higher prevalence.
Conclusions While robust national prevalence studies are sparse in Nigeria, our findings suggest 7 in every 100 pregnant women are likely to have HIV infection. These figures are consistent with reported prevalence rates in sub-Saharan African region. WHO has indicated much higher prevalence in Nigeria compared with our findings. This discrepancy could potentially be attributed to varied methodological approaches and regional focus of studies included in our review. The magnitude of the issue highlights the need for targeted efforts from local, national and international stakeholders for prevention, diagnosis, management and treatment.
- HIV & AIDS
- public health
- maternal medicine
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
Our study is the first meta-analysis that estimated pooled prevalence rates of HIV among pregnant women in Nigeria and examined variations by geopolitical zones and study characteristics.
We followed Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines and used standardised data extraction and quality assessment tools to increase the validity of our review.
More than half of the included studies were of moderate methodological quality with a high risk of selection and sampling bias.
Generalisability of our study findings may be limited as the included studies were confined to four of the six geopolitical zones in Nigeria.
Introduction
HIV infection among pregnant women has emerged as a global public health issue with serious medical, economic and social impact.1 2 Global estimates suggest that 19.2 million women were living with HIV in 2019 constituting 52% of all adults living with the infection.2 HIV infection in pregnancy has become the leading cause of mortality among women of reproductive age.3 While pregnancy itself has little or no contribution to the progression of HIV in women who are asymptomatic or those in the early stage of the infection,4 it presents substantial risks to babies, families and healthcare workers.1 The overall poor health and compromised immune capacity of women living with HIV (WLHIV), especially those in the advanced stage of the infection, may cause them to be more susceptible to increased risk of obstetrical complications and adverse perinatal outcomes, including intrauterine infections, fetal growth retardation, puerperal sepsis, ectopic pregnancy, haemorrhage, low-birth weight and preterm birth.5–11 The risk of maternal death tends to increase eightfold in pregnant women living with HIV.12 Perinatal transmission of HIV is a major challenge of the HIV/AIDS epidemic, accounting for 90% of all paediatric HIV worldwide.13
HIV prevalence is notably higher among women in sub-Saharan Africa (SSA) with women constituting 60% of all those infected with HIV, and two-thirds of the infection occurring among 15–24 years old.3 14 Although researchers have ascribed about 25% of deaths among pregnant women to HIV infection, the impact of HIV on maternal mortality in SSA is difficult to evaluate due to the lack of reliable estimates from the region.7 Nigeria accounts for 10% of the HIV/AIDS burden globally,15 and has the second highest incidence of new HIV infections among women globally.16 In 2016, HIV prevalence among women in Nigeria was about 51% (1.6 million), compared with 42% among men (1.3 million).15 Women are indicated to be more vulnerable than men to HIV/AIDS pandemic in Nigeria.17
The National HIV Sero-prevalence Sentinel Survey in Nigeria showed an increase in HIV sero-prevalence rates among pregnant women nationally from 1.8% in 1991 to 5.8% in 2001, followed by a gradual decline to 5% in 2003, 4.1% in 2010 and further to 3% in 2014.18 Of an estimated 85 450 WLHIV giving birth annually (about 4.6% of all pregnancies) in Nigeria, about 56 681 births are likely to be HIV positive.19 20 Perinatal transmission accounts for about 10% of new HIV infections annually in Nigeria.16
Nigeria is a Federal Republic comprising 36 States and its Federal Capital Territory, Abuja. The states are grouped into six geopolitical zones, the North Central, North East, North West, South West, South East and South South as shown in figure 1.21
Geopolitical zones in Nigeria.21
Variations across geopolitical zones in HIV prevalence among pregnant women is likely to occur in concurrence with the overall variations in levels of infection across the country with highest prevalence in the South-South zone (5.5%) and the lowest prevalence in the South-East zone (1.8%).22
Understanding infection prevalence rates is essential for clinicians and policymakers for the development and implementation of timely and effective interventions. While various individual studies have provided some insights about the likely prevalence of HIV among pregnant women in different parts of Nigeria, they do not provide sufficient evidence on their own to warrant appropriate action. This systematic review and meta-analysis estimated the pooled overall prevalence of HIV infection among pregnant women in Nigeria and examined variations by geo-political zones and other study characteristics.
Methods
Search strategy
The review followed the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analysis’ guidelines.23 We conducted a comprehensive search of bibliographic databases including PubMed (Medline), CINAHL, PsycINFO, Global Health, E-Journals, Academic Search Elite and Allied and Complementary Medicine Database (AMED) for studies published between 1 January 2008 and 31 December 2019. Additional sources searched included Google Scholar, authors’ institutional libraries, reference list of identified articles and grey sources such as reports, conference abstracts, presentations and proceedings.
We used a combination of text words and MeSH (Medical Subject Headings) terms to conduct the searches as follows: (‘Human immunodeficiency virus’ OR ‘HIV’) AND (prevalen* OR inciden* OR epidemiolog* OR frequen* OR occurren*) AND (pregnan* OR prenatal OR antenatal OR perinatal OR maternal) AND Nigeria. See online supplemental file 1 for a full search strategy for all databases.
Supplemental material
Screening and selection criteria
Primary observational studies published in peer-reviewed journals since 2008 were included if they reported prevalence estimates of HIV among pregnant women in Nigeria using a diagnostic/screening test.
The screening was conducted in three stages. The first and second stages involved screening of titles and abstracts respectively of all search results for relevance along with the retrieval of the full texts of all ‘included’ and ‘may be included’ articles. In stage three, a comprehensive assessment of the full-text articles was undertaken.
Data extraction and quality appraisal
A data extraction form was developed based on the guidance from the Centre for Reviews and Dissemination (CRD), University of York (https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf) and the following data were extracted and entered manually into the computer-based form: author(s), year of publication, objective of the study, study design, setting and time frame, sample definition, sampling technique and sample size, response rate, data collection methods and outcome measure, particularly prevalence rate of HIV among pregnant women. Three authors (COO, RM and MA) undertook the data extraction and SP cross-checked for accuracy.
Following data extraction, the selected studies were critically assessed for methodological quality using a modified version of ‘Guidelines for evaluating prevalence studies’.24 Two authors (COO and RM) assessed and rated the eligible studies based on three main domains: sampling, measurement and analysis. These domains were further divided into eight criteria and for each criterion, one point was given if the answer was ‘yes’, and zero points if the answer was ‘no’ with the total score ranging from 0 to 8 (Box 1). Studies with a total score of 0–2 were considered as of ‘low quality’, a score of 3–5 as of ‘moderate quality’ and a score of 6–8 as of ‘high quality’. Additionally, studies were assessed for external and internal validity using a modified risk of bias tool for prevalence studies25 and were rated as of high or low risk of bias for each component in the tool.
Assessment criteria for study quality
Sampling (maximum score=3)
Was the target population clearly defined using shared characteristics such as demographic features?
Was probability sampling used to identify potential respondents?
Was there a reporting of inclusion and exclusion criteria for sample selection?
Measurement (maximum score=4)
Was the response rate higher than 80%?
Was the data collection method standardised, including the use of diagnostic/screening test and identical methods of assessment with all the respondents?
Were the data collection method reliable, in terms of reporting the names of the manufacturer’s specification for testing kits and following test guidelines?
Were the study instruments valid by providing the names of screening and confirmation tests?
Analysis (maximum score=1)
Was the study included CIs for statistical estimates or the information needed to calculate them?
Data synthesis and analysis
We conducted meta-analysis to estimate pooled prevalence and generated forest plots for Nigeria as a whole and for individual geopolitical zones with 95% CI. I2 statistics was used to assess the heterogeneity between studies, in which I2 greater than 50% indicated substantial heterogeneity.26 We used a random-effects model to combine individual prevalence data, considering the variance that existed between individual studies.27 We conducted subgroup analyses with respect to the following study characteristics: geopolitical zone, sampling technique and sample size, length of study, study period, study quality and risk of bias. Publication bias was assessed using funnel plot and Egger’s test. The ‘Meta’ package 4.9–2 and ‘Metafor’ package 2.0–0 in R statistical software and R Studio as Integrated Development Environment were used for the meta-analysis.28
A review protocol was developed and published (PROSPERO 2019 CRD42019107037).
Public and patient involvement
No patients or public were involved in formulating the research question, defining the outcome, analysis and interpretation or writing up of results. No data were directly collected from patients during the course of the study. Where possible, results of the study will be disseminated to the public and patient community by the authors.
Results
Study characteristics
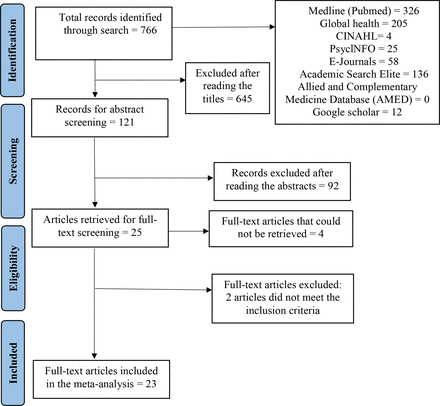
The initial search produced a total of 766 records from which 121 titles were identified following stage 1 screening. In stage 2, 92 records were excluded following abstract screening, and 29 articles were identified for full-text screening. Full texts of four articles were inaccessible. Twenty-five articles underwent stage 3 screening and 23 articles were selected29–51 after excluding two studies that did not provide the details of HIV diagnostic tools (figure 2).
Preferred Reporting Items for Systematic Review and Meta-Analysis flow diagram of the study selection process.
A total of 72 728 pregnant women from 23 studies were included in this systematic review. The women were aged 15–49 years and all the studies were conducted in healthcare facilities. Ten studies were conducted in tertiary (teaching) hospitals,30–33 35 37 38 40 42 51 five in general hospitals,29 34 41 46 47 two in antenatal care clinics43 49 and one each in the following settings: a military hospital,36 a cottage hospital,44 a Prevention of Mother to Child Transmission (PMTCT) centre,45 maternity centres across two contiguous states,48 community-based testing centres50 and a traditional birth home.39 Majority of the studies did not clearly mention the trimester in which the HIV testing was done, however, it was stated that women were tested at the time of booking.
The overall sample size in individual studies ranged from 118 to 37 464. Majority of the studies were conducted in the South-South geopolitical zone,11 29 31–33 35 36 38 39 41 44 46 followed by North-Central zone.7 31 42 43 45 47 50 51 Few studies were conducted in South-East2 37 40 and South-West2 30 48 geopolitical zones. One study included participants from three zones including South-West, South-East and North-Central.49 There were no studies from North-East and North-West geopolitical zones. Twelve studies were published after 2013 whereas 11 studies were published prior to 2013.
The majority (nine) were cross-sectional studies34 36 38 39 43–45 48 51; two studies each used prospective37 42 and retrospective designs41 46; two studies used a combination of cross-sectional and prospective designs.31 47 Among the seven studies that reported sampling methods, four used simple random sampling37 43 45 48 and the remaining used convenient sampling techniques.31 41 51 The study duration was less than a year in most of the studies10 31 34 36 37 41 43 45 48 49 51 whereas eight studies were conducted for a period of 1–3 years.29–31 35 38–40 50 Two studies were conducted for longer durations of 5 and 9 years.42 46 Twenty-one studies used two or more diagnostic tests to identify participants with HIV. The diagnostic tests included: Capillus, Genie, Determine, Star-pak, Unigold, Western blot, ELISA (Immunocombo), EIA kit and Genscreen. Nearly half of the included studies (10) were rated as of high quality and the remaining were of moderate quality (table 1).
Summary of study characteristics
The majority (20) of the studies apart from three31 49 50 examined the prevalence of HIV with respect to sociodemographic factors: age, marital status, level of education, occupation, gestational age and parity although there were variations in the way the rates were calculated. Twelve studies31–36 39 41 42 44–46 48 51 used the sample size in a particular sociodemographic variable subgroup as denominator when calculating HIV prevalence for that particular subgroup while total sample size was used in five29 30 32 37 40 and total number of study participants diagnosed as HIV positive was used in three studies.38 43 47
Seventeen studies29 30 33–39 41 42 44–48 51 reported HIV prevalence rates within various age groups ranging from 11 to >40 years. Thirteen studies32–34 36 38 39 43–48 51 reported the level of education of the study participants when reporting HIV prevalence. Only five studies reported HIV prevalence according to pregnancy trimesters.34 45–47 51 Gravidity and parity of study participants were reported in nine studies.32 36 38 39 42 45–48
Prevalence of HIV among pregnant women in Nigeria
Among the 72 728 pregnant women included, 4981 women were diagnosed as HIV positive with prevalence rates ranging from 2.4% in North-Central zone to 25.42% in South-South geopolitical zone. The overall pooled prevalence of HIV among pregnant women was 7.22% (95% CI 5.64 to 9.21; figure 3). A high degree of heterogeneity was found in included studies (Higgins I2=97.2%). The funnel plot and egger test (p=0.728) showed evidence of publication bias.
Pooled prevalence of HIV infection in pregnant women.
The pooled prevalence for individual geopolitical zones, except South-South (7.16%, 95% CI 5.07 to 10.02), showed substantial variations compared with the overall pooled prevalence. North-Central (6.84%, 95% CI 4.73 to 9.79) and South-West (6.27%, 95% CI 4.75 to 8.24) geopolitical zones had a lower estimate whereas the estimate for South-East zone (17.04%, 95% CI 9.01 to 29.86) was higher (table 2, figure 4).
Subgroup analysis of the prevalence of HIV among pregnant women in Nigeria
Pooled prevalence of HIV infection with respect to geopolitical zones.
There was no significant difference in the pooled prevalence rates between studies that used convenient sampling (7.20%) whereas studies that used simple random sampling (7.95%) showed a slightly higher prevalence rate overall (figure 5). With respect to sample size, studies that included a sample size >3297 found lower prevalence (6.07%) compared with those with a sample size <3297 (7.60%) (figure 6).
Pooled prevalence of HIV infection with respect to sampling methods.
Pooled prevalence of HIV infection with respect to study sample size.
The pooled prevalence for studies conducted in tertiary hospitals (10.24%) was higher compared with studies conducted in antenatal care clinics (3.04%). There was no significant difference in prevalence rates between studies that were carried out for >11 months (7.25%) and ≤11 months (7.32%) (table 2). The pooled prevalence was higher for studies that were conducted prior to April 2011 (8.07%) compared with studies conducted after April 2011 (5.94%) or covering April 2011 (6.92%) (table 2).
The pooled prevalence from 10 high-quality studies (n = 15 634) was higher, 8.38% (95% CI 5.22 to 13.18) compared with pooled prevalence from 13 moderate-quality studies, 6.57% (95% CI 5.23 to 8.22) (n=57 094) (figure 7). Exclusion of studies with higher risk of sampling bias lowered the prevalence estimate to 6.69% (95% CI 3.00 to 14.23). Studies with higher risk of reliability and validity of diagnostic tests reported a lower prevalence estimate of 5.02% (95% CI 3.50 to 7.14) (table 2).
Pooled prevalence of HIV infection with respect to study quality.
Differences in prevalence with respect to sociodemographic factors
Among studies that used the sample size in a particular sociodemographic variable subgroup as denominator when calculating HIV prevalence, the highest HIV prevalence rates were reported in the age groups of 41–45 years (100%),34 36–40 years (40%)34 and 40–44 years (25%).45 Among the rest, highest HIV prevalence of 6.4% and 43.33% were reported in 29–32 years and 26–30 year groups, respectively. The lowest prevalence rates were reported in ≥30 years (1.31%),44 41–46 years (0.13%)30 and ≤19 years (3.33%).38 In most studies, the prevalence of HIV was higher among participants who had no formal education.32 36 39 44 45 Two studies reported high prevalence rates among participants who had secondary (49.16%)38 and tertiary level (50%)47 education. Seven studies reported lower prevalence rates among those who completed tertiary education compared with others.32 34 36 39 46 48 51
Three studies reported higher prevalence among participants in the second trimester compared with those in the first trimester.34 47 51 Two studies reported the highest prevalence among pregnant women in the first trimester and a lower prevalence among those in the second trimester.45 46 Among nine studies that considered gravidity and parity, six studies reported higher prevalence rates among multigravida and higher parity mothers.32 36 39 42 45 48
Discussion
To the authors’ knowledge, this is the first systematic review and meta-analysis conducted to estimate the prevalence of HIV among pregnant women in Nigeria. All included studies were from four geopolitical zones of Nigeria and we found an overall pooled prevalence of 7.22% (95% CI 5.64 to 9.21), with rates ranging from 2.4% in North-Central zone to 25.42% in South-South zone. While our overall prevalence rates are consistent with prevalence rates reported from SSA region as well as individual countries in the region,52 there have been other reports indicating much higher prevalence rates in Nigeria. The WHO reported a 41% prevalence rate of HIV among pregnant women in Nigeria which is six times higher than the pooled estimate found in our study.53 This could be an indicator of a higher actual burden of the disease in the country as a whole. The overall high prevalence of HIV among pregnant women that we found in our review could mirror the high rates of HIV among women in general that has been attributed to some of the common cultural practices followed by Nigerian societies in certain areas, such as child marriage, levirate marriage and polygamy.54 Studies have also shown that HIV prevalence among pregnant women in Nigeria is higher than the prevalence rates from other developing countries such as Brazil (0.38%),55 Ethiopia (5.74%)56 and Tanzania (5.6%).57
Wide variations in prevalence rates for studies conducted in different healthcare facilities could potentially be attributed to variations in the availability of adequate testing facilities. We found the highest prevalence in tertiary hospitals, which could be due to the better availability of testing facilities compared with other primary and secondary care facilities. The variations in pooled prevalence rates with respect to study quality also need to be taken into consideration as more than half of the studies were of moderate methodological quality with a high risk of selection bias and sampling. There was substantial heterogeneity among studies due to the variations in methodological approaches including sample size, sampling approach and the characteristics of pregnant women although the information was not available in some studies.
The wide variations in prevalence between different geopolitical zones within Nigeria is in concurrence with findings from previous studies about significant differences in HIV prevalence both within and across countries in SSA.21 58–60 This has important implications for targeted preventive as well as treatment programmes and interventions in areas with high prevalence including interventions for the prevention of perinatal transmission as well as the provision of lifelong antiretroviral drugs. Based on our findings, the South-East geopolitical zone has nearly twice the rate of HIV prevalence compared with the national average which in turn indicates the need to better target prevention efforts to these areas.
The extent of heterogeneity in a meta-analysis determines the generalisability of study findings to the entire study population. A high degree of heterogeneity (Higgins I2=97.2%), both in the pooled and subgroup analyses, indicated substantial variations between included studies,26 making it challenging to generalise the findings to the entire pregnant WLHIV in Nigeria. The variations in methodological approaches including differences in study settings, sampling methods, sample size and the diagnostic/screening procedures as well as the overall study quality could have all potentially contributed to the high degree of heterogeneity observed in our review.
Strengths and limitations
The rigorous methodological approach adopted in our study makes our findings valid and reliable. However, the prevalence estimates may not be generalisable to the whole of Nigeria as included studies were from four geopolitical zones of Nigeria and there was a high degree of heterogeneity among the included studies. More than half of the included studies were of moderate methodological quality with a high risk of selection and sampling bias. We were unable to conduct a regression analysis to analyse the effect of study level factors, such as study quality and geopolitical zone, due to the lack of adequate number of studies (<10) for each category within the particular variable as well as the high level of heterogeneity found among the included studies.
Conclusion
Our findings imply that HIV infection is a significantly prevalent issue among pregnant women in Nigeria. Determining an overall, synthesised accurate national prevalence rate based on existing evidence presents a challenge due to the lack of evidence from some geopolitical zones, and the wide-ranging and, in many cases, problematic methodological approaches adopted by some studies. While new cases of HIV have gradually decreased globally since its peak in 1999, prevalence of HIV continues to be the highest in southern SSA and it has been indicated that HIV will continue to be a major threat to public health for years to come.61 Our findings, therefore, have important implications for planners, policymakers, academics and researchers in medical and public health arenas both in Nigeria as well as in SSA region towards developing appropriate preventive, diagnostic and treatment interventions including the provision of lifelong antiretroviral drugs to all WLHIV as part of PMTCT services regardless of CD4 count (which indicates the level of HIV in the body) as recommended by WHO guidelines.62 Future research should employ scientifically rigorous methodological approaches to derive accurate national prevalence estimates and to make comparisons with other countries in the region and internationally. The observed variations in prevalence with respect to various diagnostic tests highlights the importance of having a gold-standard diagnostic tool. There is also a need for a more in-depth understanding of the associated cultural, social and environmental factors. Qualitative studies can be of great value in this respect. Progress on all these fronts will boost the development of policies and practice guidelines to effectively tackle the issue.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
Ethics approval was sought from the Ethics Committee of the Institute for Health Research, University of Bedfordshire (Ref: IHREC838).
Acknowledgments
The first author (COO) was supported by the Voice of the Last Days Ministry (Nigeria). Salaries of the other team members were provided by employing institutions.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @shubyputhussery
COO, RM, MA and SP contributed equally.
Contributors COO and SP designed the study and SP oversaw its implementation. COO and RM did the searches, study selection, data extraction and quality appraisal, and SP cross-checked for accuracy and resolved any differences. MA developed and conducted the meta-analyses and developed the tables. RM and SP wrote the manuscript. All authors reviewed and approved the final draft of the manuscript before submission. SP is responsible for the overall content as the guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.