Article Text
Abstract
Objectives Randomised controlled trial of the effect of a perineural infusion of levobupivacaine on moderate/severe phantom limb pain 6 months after major lower limb amputation.
Setting Single-centre, UK university hospital.
Participants Ninety patients undergoing above-knee and below-knee amputation for chronic limb threatening ischaemia under general anaesthesia. Exclusion criteria were patients having surgery under neuraxial anaesthesia; inability to operate a patient-controlled analgesia device or complete a Visual Analogue Scale; amputation for trauma or malignancy; or contraindication to levobupivacaine.
Interventions Either levobupivacaine 0.125% or saline 0.9% (10 mL bolus, infusion of 8 mL/hour for 96 hours) via a sciatic or posterior tibial nerve sheath catheter placed under direct vision during surgery.
Primary and secondary outcome measures The primary outcome measure was the presence of phantom limb pain, residual limb pain and phantom limb sensations up to 6 months after amputation. Secondary outcome measures included early postoperative pain and morphine requirements after surgery.
Results Data from 81 participants were analysed; 6-month follow-up data were available for 62 patients. Pain and morphine requirements varied widely before and after amputation in both groups. The incidences of moderate/severe phantom limb pain, residual limb pain and phantom limb sensations were low from 6 weeks with no significant differences between groups in phantom limb pain at rest (OR 0.56, 95% CI 0.14 to 2.14, p=0.394) or movement (OR 0.58, 95% CI 0.15 to 2.21, p=0.425) at 6 months. Early postoperative pain scores were low in both groups with no between-group differences in residual limb pain or phantom limb sensations (rest or movement) at any time point. High postoperative morphine consumption was associated with worsening phantom limb pain both at rest (−17.51, 95% CI −24.29 to −10.74; p<0.001) and on movement (−18.54, 95% CI −25.58 to −11.49; p<0.001). The incidence of adverse effects related to the study was low in both groups: postoperative nausea, vomiting and sedation scores were similar, and there were no features of local anaesthetic toxicity.
Conclusions Long-term phantom limb pain, residual limb pain and phantom limb sensations were not reduced significantly by perineural infusion of levobupivacaine, although the study was underpowered to show significant differences in the primary outcome. The incidence of phantom limb pain was lower than previously reported, possibly attributable to frequent assessment and early intervention to identify and treat postoperative pain when it occurred. There were large variations in postoperative pain scores, high requirements for analgesics before and after surgery and some problems maintaining recruitment and long -term follow-up. Knowledge of these potential problems should inform future research in this group of patients. Further work should investigate the association between perioperative morphine requirements and late phantom limb pain.
Trial registration numbers EudraCT 2007-000619-27; ISRCTN68691928.
- Adult anaesthesia
- Pain management
- PAIN MANAGEMENT
- VASCULAR SURGERY
- ANAESTHETICS
Data availability statement
Data are available on reasonable request. The data generated during and/or analysed are available from the corresponding author on reasonable request. Some trial data are also available via the EudraCT and ISRCTN websites.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is the largest double-blind randomised placebo-controlled study of the effect of perineural local anaesthetic infusion on long-term phantom limb pain after major lower limb amputation.
In contrast to previous studies, we differentiated between phantom limb pain, residual limb pain and non-painful phantom limb sensations.
The study included a rigorous protocol to detect and treat pain after amputation, in line with modern pain management practice.
There was good adherence to the study protocol, with a higher proportion of recruited patients completing 6-month follow-up compared with similar previous studies.
Enrolment reduced over time, and because we were unable to include multiple sites, the planned recruitment target was not reached.
Introduction
Peripheral arterial disease affects one in five people aged over 60 years in the UK.1 Over 3500 patients with chronic limb threatening ischaemia (CLTI) require emergency revascularisation every year.2 Despite these aggressive attempts at limb salvage via reconstructive surgery or angioplasty, approximately 3000 patients in the UK undergo major lower limb (above and below knee) amputation annually.2 3
Major amputation is a significant cause of morbidity and mortality.3 Pain after amputation significantly affects quality of life and postoperative recovery.4 5 Pain that persists after amputation typically occurs as residual limb pain at the surgical incision site, phantom limb pain or both.6 7 Non-painful phantom limb sensations also occur commonly, but are not usually troublesome.6
Phantom limb pain is a well-described but varied phenomenon with a commonly reported incidence of 32%–80% of amputees.7–10 The onset of phantom limb pain is often within days of surgery, and severity usually decreases over time.7 11 However, once established phantom limb pain is very difficult to manage, and has significant adverse effects on quality of life, rehabilitation and the ability to mobilise using a prosthetic limb.7 11 12
Several techniques including regional anaesthesia, transcutaneous nerve stimulation or analgesics such as ketamine have been investigated for the prevention or alleviation of phantom limb pain and residual limb pain. However, the data are conflicting, most studies have been small and have often not distinguished between residual limb pain, phantom limb sensations and true phantom limb pain.13–16 Although some studies have shown reductions in phantom limb pain and residual limb pain from preoperative and postoperative epidural analgesia, the largest single trial found no benefit.13 17 In addition, many patients presenting for major amputation are receiving a variety of antiplatelet and anticoagulant medications for primary or secondary prevention of cardiovascular disease, or in an attempt at limb salvage.18 In these patients, neuraxial anaesthesia is relatively contraindicated and a perineural local anaesthetic infusion may be more appropriate.19
Several studies have investigated the effect of perineural local anaesthetic infusion on postoperative pain after amputation, based on the rationale that prevention of central sensitisation will reduce or prevent the subsequent development of chronic pain syndromes.6 8 However, many of these studies have reported conflicting results, and there is no definite consensus on the efficacy of the technique to prevent phantom limb pain.10 20–28 Most studies of perineural anaesthesia were not controlled trials or used retrospective controls.21 22 24 26 29 Furthermore, one did not distinguish between phantom limb sensations, phantom limb pain and residual limb pain, and the largest study (198 patients) was retrospective and did not assess patients for phantom limb pain.23 26 Variations in definitions and measurement of pain after amputation are present throughout the existing literature. A recent randomised controlled trial demonstrated that perineural infusion of ropivacaine significantly reduced residual limb pain in the early postoperative period, but there was no decrease in intensity of phantom limb pain after 12 months.25
Given the effect of phantom limb pain on quality of life and rehabilitation, and the simplicity and cost-effectiveness of perioperative nerve sheath infusions, it is important to establish their efficacy on pain after lower limb amputation. This has recently been highlighted as a top research priority within this group of patients.30 Therefore, we conducted a prospective, double-blind, randomised clinical trial with the hypothesis that perineural local anaesthetic infusion would reduce the incidence and intensity of phantom limb pain. Our primary aim was to assess the effect of a perineural infusion of levobupivacaine on phantom limb pain, residual limb pain and phantom limb sensations 6 months after amputation. Secondary aims were the effect of the infusion on acute postoperative residual limb pain, morphine consumption and time to fitness to hospital discharge.
Methods
Patient and public involvement
There was no involvement of patients or the public in the development of the trial protocol.
Study design and patients
The study was reported via Consolidated Standards of Reporting Trials statement guidelines.31 All patients admitted to the vascular unit at Leicester Royal Infirmary who needed major lower limb amputation for CLTI were eligible for the study. Two hundred and four patients were screened for inclusion between October 2007 and October 2013 (figure 1). Patient enrolment began on 1 October 2007. Exclusion criteria were: patients unfit for general anaesthesia; patients undergoing surgery under regional anaesthesia, inability to operate a patient-controlled analgesia device (PCA) or to complete a Visual Analogue Scale (VAS); amputation for trauma or malignancy; a known contraindication to levobupivacaine; or inability to provide informed consent. Patients meeting the inclusion criteria were recruited after surgeons had decided that amputation was needed and written informed consent for the study was obtained (SB and JPT). Baseline characteristics including age, sex, comorbidities, current medications and use of analgesics were recorded before surgery.
Consolidated Standards of Reporting Trials Diagram of patient flow through the study. PCA, patient-controlled analgesia.
Randomisation
Patients were randomised (1:1, blocks of 10) to receive either levobupivacaine (group L) 0.125% (Chirocaine, Abbott Laboratories, Maidenhead, UK) or 0.9% saline (group S) for perineural infusion. Treatment randomisation was allocated via an internet link (with blinding maintained) by the Clinical Trials Support Unit, University of Nottingham, UK. An unblinded randomisation email notification was then sent to the hospital pharmacy indicating the treatment required for preparation and concealment in a protective package, so the study team and all clinical staff involved in the patients’ care and follow-up were blinded to the treatment group.
Study procedure
All patients received standardised general anaesthesia, comprising intravenous propofol titrated to loss of consciousness, maintenance with nitrous oxide and isoflurane in oxygen and intravenous morphine 0.1–0.15 mg/kg and paracetamol 1 g. During surgery, the surgeon identified the sciatic nerve under direct vision (patients undergoing above-knee amputation (AKA)) The sciatic nerve divides at the knee and so in patients undergoing below knee amputation its major branch, the posterior tibial nerve was identified directly. For all patients the sciatic or posterior tibial nerve was then transected 4–5 cm proximal to the wound and a 16 G epidural catheter was inserted at least 5 cm alongside the nerve within the nerve sheath. The catheter was then sutured to the nerve sheath and surrounding soft tissue by an absorbable Vicryl stay suture to ensure it stayed in position after surgery. The catheter was also secured into position at the skin using a silk suture and adhesive strips. After 4 days, the stay suture was excised from the skin, and the catheter was withdrawn by gentle retraction from the amputation site. All surgeons received appropriate training in standardised placement and fixation of the perineural catheter before performing the procedure. A bolus of levobupivacaine 0.125% 10 mL (group L) or saline 0.9% 10 mL (group S) was given followed by a continuous infusion (via an electronic infusion pump) of the same at a rate of 8 mL/hour for 4 days. Levobupivacaine was used because of its lower potential for cardiotoxicity compared with other local anaesthetic agents.
Pain at rest and movement was recorded by the patient using a manual VAS (0–100 mm). Pain scores were categorised as no pain (0–4 mm); mild pain (5–39 mm); moderate pain (40–69 mm) and severe pain (70–100 mm).32 Because of the sensitivity of the scale, and the potential for inaccurate transcribing by frail elderly patients after surgery, VAS ≤4 mm was classified as no pain.33 Non-painful phantom limb sensations were recorded as either present or absent, and phantom limb sensation intensity was recorded using a Verbal Rating Scale (VRS) (range 0–10). VRSs were categorised as none (0); mild (1–3); moderate (4–6) and severe (7–10)7–10 sensations.
Baseline VAS scores were measured within 6 hours before surgery. After amputation, VAS scores were recorded hourly for the first 4 hours, 4-hourly up to 24 hours, and then 12-hourly up to 96 hours. From 24 hours onwards, patients were asked to differentiate between residual limb pain and phantom limb pain. Pain scores were further recorded after 1 week, 3 weeks, 6 weeks, 3 months and 6 months. The presence and intensity of non-painful phantom limb sensations were recorded at the same time points.
Postoperative analgesia
After surgery, all patients received morphine (1 mg bolus, 5 min lockout via the PCA device) with additional intravenous morphine boluses given by the postoperative recovery room nurse if required. The PCA morphine bolus was increased to 2 mg in patients who reported a VAS >70 mm scale and had received >20 mg morphine intravenous to treat acute pain in the postoperative recovery room. PCA morphine was continued for at least 48 hours after surgery, and if VAS >35 mm at 48 hours, it was continued up to 96 hours postoperatively. After discontinuation of PCA morphine, patients received regular oral analgesia (codeine phosphate 60 mg 6-hourly or tramadol 100 mg 6-hourly). Oral morphine was given on request for breakthrough surgical wound pain. Drugs being taken for analgesia before surgery (long-acting opioids, gabapentin, amitriptyline) were continued at the same dose for at least 48 hours. Following discontinuation of PCA, long-term opioid therapy was titrated down in accordance with the patient’s clinical condition. Gabapentin (300 mg daily up to a maximum of 1.8 g) was started as rescue medication if VAS >70 mm at rest 48 hours after surgery or if the patient complained of definite phantom limb pain. If phantom limb pain persisted during follow-up, patients were referred to the hospital’s chronic pain specialists.
Study outcomes
The primary outcome measures were:
The presence (or absence) of moderate or severe (VAS ≥40 mm) phantom limb pain at rest and on movement 6 months after amputation.
Improvement of phantom limb pain and residual limb pain from the baseline at rest and on movement during the 6-month follow-up.
The presence of moderate to severe phantom limb sensations (VRS ≥4) during the 6-month follow-up.
Secondary outcomes were:
Early postoperative pain, measured as the change in acute postoperative residual limb pain from the baseline at rest and on movement in the first 96 hours after surgery.
Early postoperative morphine requirements in the first 96 hours after surgery. Bioequivalent morphine consumption was calculated by combining PCA with oral morphine requirements (using a conversion factor of 0.33 for oral morphine).
Late effects on mood, physical disability and quality of life, measured using the Late Life disability index (quality of life and disability), the 36-item Short Form health survey (SF-36) inventory, (pain and disability) the McGill pain questionnaire (pain) and the University of Leicester Amputee questionnaire (pain and disability) (data not presented here).
Time from surgery to fitness for discharge from hospital.
Sample size calculation
The study size was based on our previous pilot study in a similar cohort of patients, in which 1 out of 5 (20%) patients receiving a sciatic nerve sheath infusion of bupivacaine reported phantom limb pain at 6 months, compared with 5 out of 6 (83%) in those receiving a placebo infusion.34 Based on these data and the incidence of phantom limb pain reported in the literature, we calculated that 35 patients per group would be required to show a 3-fold reduction in the incidence of phantom limb pain at 6 months from a conservative estimate of 50%–17% (β=0.2, α=0.05). The power calculation was revised after inspection of the blinded data by the trial data monitoring committee found the overall incidence of phantom limb pain was lower than anticipated. The revised calculation showed that 66 patients per group completing the 6 month follow-up with evaluable data would be required to detect a 3-fold reduction in the incidence of phantom limb pain at 6 months. The protocol was amended accordingly and changed to permit the inclusion of other centres, to increase overall study numbers.
Statistical analysis
All data were analysed on per-protocol basis. Primary and secondary outcomes were assessed using a range of linear and logistic regression models adjusted for preoperative VAS and morphine requirements (see online supplemental material). All models were evaluated for other predictors such as age, sex, presence of diabetes and types of operation and retained statistically significant (p<0.05) predictors in the final model. No imputation was performed for missing data. Full details of the statistical analysis are presented as online supplemental material. All statistical analyses were conducted in SPSS V.26.0 (IBM) and the R software V.3.6 (R Foundation for Statistical Computing, Vienna, Austria) environments. Categorical variables are presented as frequencies (%), and numerical variables are summarised as mean (SD) or median (IQR) as appropriate. For more detailed description of the statistical analyses, see online supplemental methods.
Supplemental material
Results
Two hundred and four patients were screened for inclusion into the study between October 2007 and October 2013. Ninety patients met the eligibility criteria and provided written consent. As the number of patients undergoing major amputation had decreased in our hospital, and because we were unable to extend recruitment to other centres, recruitment was terminated in 2013, on the advice of the data monitoring committee, before the planned enrolment of 132 patients. Overall, data from 81 patients were analysed (9 exclusions in the perioperative period, figure 1), with 6-month follow-up data available for 62 patients. The median age of participants was 71 (range 41–94) years, 63% (n=51) were males and 56% (n=45) had an AKA. Groups L and S were well matched for age, sex, comorbidities, preoperative morphine consumption and duration of preoperative pain (table 1).
Baseline characteristics, expressed as N (%) or median (range) except for age, which is presented as mean (range)
Phantom limb pain
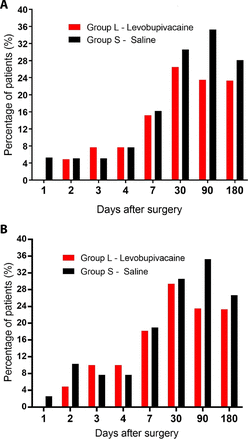
There was a wide variation in the incidence and severity of phantom limb pain after surgery in both groups (table 2). The incidence and severity of phantom limb pain at rest and on movement was similar between both groups at all postoperative time points (table 2, online supplemental tables 1 and 2). The overall peak incidence of moderate/severe phantom limb pain at rest across both groups was 29.4% (n=20/68) after 3 months, while the peak incidence of moderate/severe phantom limb pain on movement across both groups was 30.0% (n=21/70) after 6 weeks (online supplemental tables 1 and 2).
Supplemental material
Visual Analogue Pain Scores on rest and movement (scale 0–100) between 24 hours and 6 months after amputation, presented as median (IQR)
For the primary outcome, there was no significant difference in the presence of moderate/severe phantom limb pain at rest (OR: 0.56, 95% CI: 0.14 to 2.14; p=0.394) or phantom limb pain at rest on movement (OR: 0.58, 95% CI: 0.15 to 2.21; p=0.425) between the treatment groups at 6 months (table 3, online supplemental table 3). When accounted for morphine consumption, the mean VAS at rest and movement at 6 months were approximately 42%–44% lower in group L compared with group S (table 3, online supplemental table 3). However, the effect of the treatment group was not statistically significant for phantom limb pain at rest (−0.92, 95% CI −1.92 to 0.09; p=0.073) and phantom limb pain on movement (−0.98, 95% CI 1.99 to 0.03; p=0.056) (table 3, online supplemental table 4). The improvement in VAS scores for phantom limb pain at rest (11.95, 95% CI −3.76 to 27.66; p=0.133) and phantom limb pain on movement (10.83, 95% CI −5.23 to 26.88; p=0.182) at 6 months compared with baseline was also not statistically significant (table 3, online supplemental table 5, online supplemental figure 1).
Supplemental material
ORs and estimates of mean differences between groups (L vs S) and corresponding 95% CIs for primary and secondary outcomes at rest and on movement
The incidence of moderate or severe phantom limb pain at rest and on movement (online supplemental tables 1 and 2) increased from the few days after amputation (figure 2). Phantom limb pain severity increased in the first 6 months after surgery in both groups at rest (−22.10, 95% CI −30.69 to −0.18); p<0.001) and movement (−21.95, 95% CI −30.68 to −13.23; p<0.001) (table 4). When adjusting for all time points, there was no significant improvement in phantom limb pain at rest (1.09, 95% CI −2.06 to 4.23; p=0.498) and phantom limb pain on movement (0.87, 95% CI −2.33 to 4.06; p=0.595) scores between groups (tables 3 and 4).
Incidence of moderate and severe phantom limb pain (VAS ≥40) at each time point after amputation at (A) rest and (B) movement. VAS, Visual Analogue Scale.
Summary of estimates and corresponding 95% CIs of linear model using generalised least squares for improvement of phantom limb pain at rest and movement for all time points (1 day to 6 months after amputation)
High postoperative morphine consumption compared with no use of analgesics showed worsening phantom limb pain at rest (−17.51, 95% CI −24.29 to −10.74; p<0.001) and on movement (−18.54, 95% CI −25.58 to −11.49; p<0.001) up to 6 months (table 4). Higher preoperative pain scores were positively associated with the improvement in phantom limb pain at rest (1.00, 95% CI 0.95 to 1.04; p<0.001) and on movement (0.98, 95% CI 0.93 to 1.02; p<0.001) in the 6 months after amputation (table 4).
Residual limb pain
Despite the wide variation between patients, residual limb pain scores decreased over time after surgery (p<0.001) and were low in most patients from 1 week after amputation (table 2). Early postoperative pain scores were consistently lower than pain before surgery (online supplemental table 6). However, there residual limb pain severity at rest and on movement was similar between the groups (table 2). Similarly, there were no differences between groups in the improvement of residual limb pain at rest (2.00, 95% CI −1.46 to 5.46; p=0.257) or on movement (−0.97, 95% CI −5.70 to 3.76; p=0.687) (table 3, online supplemental table 7, online supplemental figures 2 and 3).
Supplemental material
Supplemental material
Patients with a higher daily intake of analgesics in the postoperative period experienced significant worsening in residual limb pain at rest (−17.40, 95% CI −24.44 to −10.35; p<0.001) and on movement (−21.42, 95% CI −30.45 to −12.39; p<0.001) compared with those who did not take any analgesia (online supplemental table 7). Patients with higher pain scores before surgery experienced greater improvements in residual limb pain at rest (0.98, 95% CI 0.93 to 1.03; p<0.001) and on movement 1.05, 95% CI 0.97 to 1.13; p<0.001) after amputation compared with those reporting less preoperative pain (online supplemental table 7). There was no evidence that the improvement of acute residual limb pain at rest (3.16, 95% CI −0.88 to 7.20; p=0.125) and on movement (1.43, 95% CI −3.41 to 6.27; p=0.563) differed between treatment groups regardless of any postoperative time points (table 3, online supplemental table 8).
Phantom limb sensations
The presence of phantom limb sensations increased with time after amputation (p<0.001) (online supplemental table 2). There were no significant differences between the study groups in the presence of phantom limb sensations (OR: 1.13, 95% CI 0.60 to 2.14; p=0.708) or its intensity (OR: 0.82, 95% CI 0.46 to 1.47; p=0.513) at 6 months (table 3). There was also no effect of preoperative pain on the incidence and severity of phantom limb sensations.
Requirements for postoperative analgesics
Morphine requirements in the early postoperative period were similar in both groups (online supplemental table 9). There was no significant difference in mean bioequivalent combined morphine consumption at 96 hours between groups L and S after adjustment for baseline pain at rest (−0.13, 95% CI −0.55 to 0.30; p=0.567) and movement (−0.12, 95% CI −0.55 to 0.31; p=0.581) (table 3). More patients in group S (n=14) received rescue analgesia with gabapentin after 1 week than group L (n=9). However, there was no significant difference overall in rescue medication use between the two groups at any postoperative time point (online supplemental table 10).
Time to fitness for hospital discharge
Time to fitness for discharge was similar between the two groups (group L, 12 (7–15.75) days; group S 12 (8–17) days) (p=0.804).
Adverse events and complications
In the perioperative and early postoperative period, nausea, vomiting and sedation scores were similar (online supplemental table 11). No symptoms or signs of local anaesthetic toxicity were recorded in either group. In the 6 months of follow-up, seven patients in group L required revision surgery (six for non-healing wounds and one underwent amputation of the contralateral leg), six died in the follow-up period and three developed wound infections. In group S, two required revision surgery (for non-healing wounds), seven died in the follow-up period and seven developed wound infections. There were no problems related to perineural catheter insertion or malfunction reported in either group. The incidence of other serious adverse events was similar in both groups (online supplemental table 12).
Discussion
In this study, both the incidence and severity of phantom limb pain after amputation were generally low, and residual limb pain scores were low from 6 weeks after surgery. Perineural infusion of levobupivacaine 0.125% did not significantly reduce phantom limb pain, residual limb pain or phantom limb sensations in the first 6 months after major lower limb amputation compared with placebo. There was wide variation in the severity of pain in the ischaemic limb and a greater than 100-fold difference in requirements for opioid analgesics before surgery. Higher postoperative morphine requirements were associated with higher pain scores in the first week after amputation and an increased incidence of phantom limb pain at 6 months.
Several previous studies of perineural local anaesthetic infusions have found reductions in phantom limb pain after lower limb amputation.21 24 26 However, a recent randomised trial demonstrated no benefit.25 Other studies have shown reductions in early postoperative residual limb pain and morphine consumption with a perineural local anaesthetic infusion.10 23 25 29 Much of the existing literature has been limited by complex issues, which affects pain research in this group, including large variations in postoperative pain scores, high requirements for opioid and other analgesics, difficulties in recruitment and significant levels of attrition during prolonged follow-up.28
In patients receiving perineural local anaesthetic, the reported incidence of phantom limb pain ranges from 18% to 88%, although these studies often included younger patients undergoing amputation for malignancy and trauma.22 24 35 A recent randomised controlled trial of patients with CLTI reported an incidence of 53% 1 year after amputation, with 19% describing this as severe.25 In our trial, only 23% of patients across both groups reported moderate-to-severe phantom limb pain at 6 months, which was much less than anticipated and previously described. The lower degrees of pain reported overall may have contributed to the lack of differences between groups, though in several sensitivity analyses that modelled the change in phantom limb pain compared with baseline pain scores, the improvement in phantom limb pain was consistently greater in patients receiving levobupivacaine. While this provides some evidence of a possible effect of the perineural infusion, this was not statistically significant.
There are several possible explanations for the differences between our and previous studies, relating to variations in study methods, design, populations and cut-offs for reporting phantom limb pain.6 9 First, we used a rigorous protocol of pain and sensation assessment, regular follow-up from a research nurse dedicated to the study, and early intervention to treat pain when needed. All patients received PCA morphine for 48–96 hours after surgery; any patients reporting any degree of phantom limb pain in the study received gabapentin as rescue medication; if phantom limb pain persisted, an early referral was made to chronic pain specialists for further management. Early and effective pain management represents modern practice and may prevent or attenuate the development of spinal cord sensitisation and chronic neuropathic pain.36 In addition to treating acute postoperative pain, we believe our rigorous study design contributed to the lower incidence of phantom limb pain due to frequent assessment, effective analgesia, and early intervention with rescue medication. This study may have obscured any possible treatment effect compared with previous data, as previous studies might have used less rigorous protocols for assessing and treating phantom limb pain.24–26 For these reasons, it is difficult to make firm conclusions from this study that are clearly applicable to the wider population.
Second, in contrast to some previous studies, we made rigorous attempts to distinguish between phantom limb pain, residual limb pain and phantom limb sensations. We noted that both patients and clinical staff who were not part of the study team often misunderstood the distinction between phantom limb pain and phantom limb sensations (such as itching, the amputated leg being present, or neuropathic sensations). On review by the study nurse, some patients reported phantom limb pain when they were actually experiencing non-painful sensations that were not troublesome and did not require additional analgesia. We specifically enquired about these non-painful sensations and distinguished them in our analysis from true phantom limb pain. Therefore, unless previous studies specifically made this differentiation, they might have overestimated the incidence of true phantom limb pain.
Third, there was no significant reduction in early postoperative pain or morphine requirements in patients who received the perineural infusion, contrasting to previous studies in this area, although the study was underpowered to detect this10 25 This lack of difference between groups may relate to the higher pain scores and morphine requirements, and the lower proportion receiving gabapentin therapy before amputation, in group L. This highlights a possible trend towards worse pain and less effective pain control in group L before surgery.
Furthermore, our study involved patients with CLTI. The duration and severity of pain before surgery, and early postoperative morphine requirements varied widely in both groups. Preoperative pain and morphine requirements across both groups ranged from 5 mg to 645 mg daily. Many patients with CLTI have significant long-term pain, prolonged high analgesic requirements and are likely to have developed opioid tolerance. This may have confounded our results and made any additional effect of perineural levobupivacaine difficult to demonstrate. In common with similar studies, several patients did not complete the follow-up period. This emphasises the difficulties and challenges associated with studying patients after amputation. These data suggest that future research in this area should focus on larger multicentre studies and use network or registry approaches.
Finally, the effect of sciatic or posterior tibial nerve blockade alone on central sensitisation after major amputation may be limited. Others have suggested additional femoral nerve blockade may improve efficacy.22 Furthermore, other postoperative factors, such as neuroma formation or inadequate surgical cover at the stump site, can contribute to significant pain.5 37 Therefore, perioperative neural blockade alone is perhaps unlikely to abolish significant residual limb pain or phantom limb pain, or reduce morphine requirements in all patients.
The relationship between preoperative opioid use and greater postoperative pain is well established.38 Pain after amputation is more severe in patients with greater of degrees of preoperative and perioperative pain.39 40 Our findings reflect this: though there was no correlation between early and late postoperative pain scores, this study demonstrated a strong association between high perioperative opioid requirements and with higher acute residual limb pain scores and the development of phantom limb pain. This may reflect the development of opioid-induced hyperalgesia, and highlights the importance of a multimodal approach with frequent assessment and targeted strategies for perioperative pain management.6 38 41
We also noted that higher preoperative pain scores were associated with a greater degree of improvement in phantom limb pain and residual limb pain overall. This may be explained by patients with higher baseline pain having more potential for a reduction in pain, compared with those who had relatively low baseline scores.
von Plato et al published a randomised controlled trial of a perineural local anaesthetic infusion on pain after amputation that had greater initial recruitment numbers.25 However, the current study is the largest that has the prevention of long-term phantom limb pain as the primary outcome, and fewer lost to follow-up meant that our study cohort after 6 months was greater. Strengths of our study include its randomised double-blinded design; and that the trial had one dedicated research nurse, who performed almost all visits and assessments. Thus, interobserver variability was reduced, and both adherence to the study protocol and numbers completing 6-month follow-up were high. Also, surgery and placement of the perineural catheter was performed by a small number of surgeons, and all postoperative care was on the same ward, so variability in care was minimised.
The study was limited by low levels of recruitment in the latter years, and we were unable to extend recruitment to multiple centres, so that the recruitment target was not reached. Hence, the trial was underpowered to demonstrate significant differences in the primary outcome. The findings may not apply to others undergoing major amputation for other indications such as younger patients after trauma, in whom preoperative chronic pain and opioid use are likely to be lower, and the psychological implications different. Though several years have passed since data collection ceased, our study protocol of early effective multi-modal pain management reflects modern practice.
Future research in this area should focus on larger, multicentre studies with specific distinctions of phantom limb pain and non-painful phantom limb sensations. It may be beneficial to stratify for baseline pain, preoperative analgesics and other potential confounders such as the presence of diabetes. It is important to consider a multimodal approach as part of the treatment strategy before and after amputation. Combined sciatic and femoral nerve blockade with or without neuraxial anaesthesia also warrants consideration.
Conclusions
In conclusion, the incidence and intensity of phantom limb pain, residual limb pain and phantom limb sensations were low after major lower limb amputation for CLTI. Perineural infusion of levobupivacaine 0.125% was not superior to a perineural infusion of saline, although the study was underpowered to assess the primary outcome. The low incidence of phantom limb pain in this study may have resulted from strict differentiation between phantom limb pain, residual limb pain and non-painful phantom limb sensations, in contrast to some previous studies, as well as early intervention to identify and treat postoperative pain when it occurred. Pain after amputation varies widely, and a single optimum strategy for postoperative analgesia and prevention of phantom limb pain might be unrealistic.
Data availability statement
Data are available on reasonable request. The data generated during and/or analysed are available from the corresponding author on reasonable request. Some trial data are also available via the EudraCT and ISRCTN websites.
Ethics statements
Patient consent for publication
Ethics approval
The study was approved by the Medicines and Healthcare Regulatory Agency (ref. 21275/0233/001-0001) and the Trent Research Ethics Committee (07/H0405/42). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We would like to acknowledge the assistance of Dr M Viskaduraki, University of Leicester, UK for additional statistical advice; and both Prof D.J. Rowbotham and Mr M. McCarthy, University Hospitals of Leicester NHS Trust, for advice on the trial protocol.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Study conception and design: JPT. Acquisition, analysis and interpretation of data: JPT, SB, MN, WH and LC. Specialist statistical analysis: MN. Writing of the first draft of the manuscript: JPT, SB, WH and MN. Revision of the manuscript critically for important intellectual content: JPT, WH, MN and LC. Guarantor: JPT. All authors read and approved the final version of the manuscript.
Funding This work was supported by Action Medical Research (grant reference: AP1178) and the Association of Anaesthetists of Great Britain and Ireland (AAGBI) Departmental Project Grant 2006 (no grant number).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.