Article Text
Abstract
Objective Investigate risk for falls, fractures and syncope in older adult patients treated with nortriptyline compared with paroxetine and alternative medications.
Design Retrospective cohort study.
Setting The electronic medical record and prescription drug database of a large integrated healthcare system in Southern California.
Participants Ambulatory patients, age ≥65 years diagnosed with depression, anxiety disorder or peripheral neuropathy, dispensed one or more of ten study medications between 1 January 2008 and 31 December 2018.
Main outcome measures HR for falls, fractures and syncope with exposure to study medications adjusted for patient demographic variables and comorbidities.
Results Among 195 207 subjects, 19 305 falls, 15 088 fractures and 11 313 episodes of syncope were observed during the study period. Compared with the reference medication, nortriptyline, the adjusted HRs (aHRs) for falls were statistically significantly greater for: paroxetine (aHR 1.48, 95% CI 1.39 to 1.57), amitriptyline (1.20, 95% CI 1.08 to 1.33), venlafaxine (1.44, 95% CI 1.34 to 1.56), duloxetine (1.25, 95% CI 1.12 to 1.40), fluoxetine (1.51, 95% CI 1.44 to 1.59), sertraline (1.53, 95% CI 1.44 to 1.62), citalopram (1.61, 95% CI 1.52 to 1.71) and escitalopram (1.37, 95% CI 1.21 to 1.54), but not gabapentin (0.95, 95% CI 0.89 to 1.02). For fractures, compared with nortriptyline, aHRs were significantly greater for: paroxetine, venlafaxine, duloxetine, fluoxetine, sertraline, citalopram, escitalopram and gabapentin, with aHRs ranging from 1.30 for gabapentin to 1.82 for escitalopram; risk was statistically similar for amitriptyline. For syncope, the aHRs were significantly greater for: paroxetine, venlafaxine, fluoxetine, sertraline and citalopram, with aHRs ranging from 1.19 for fluoxetine and paroxetine up to 1.30 for citalopram and sertraline; risk was similar for amitriptyline, duloxetine, escitalopram and gabapentin.
Conclusions Compared with therapeutic alternatives, nortriptyline was found to represent a lower risk for falls, fractures and syncope, versus comparator medications, except for a few instances that had equivalent risk. The risk for these adverse events from paroxetine was comparable to the alternative medications.
- primary health care
- aged
- geriatric medicine
- adverse events
Data availability statement
No data are available. The data underlying this article are not shared due to the privacy policy of data providers.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study was conducted in a large, diverse integrated healthcare system of 4.8 million members using a comprehensive electronic medical record and prescription drug database.
Study medication use reflects evidence-based practice by thousands of individual physicians within the large healthcare system.
The study medication cohorts were large, highly comparable and the duration of therapy substantial, with all eligible patients included with adjustment for many clinically important comorbidities and concomitant medications.
The identification of diagnoses and adverse drug events was based on International Classification of Diseases-9 (ICD-9) and ICD-10 coding.
The possibility of minor residual confounding in our results cannot be excluded.
Introduction
Considerable research has been conducted over the past 30 years investigating the risk of adverse drug events in older adult patients.1–7 Due to age-related changes in drug pharmacodynamics and pharmacokinetics, with increased prevalence of diminished function of organ systems, the balance of benefit versus risk for many medications is adversely shifted in an age-related manner in older patients.8 9 There have been expert and critical reviews and compilations of high-risk medications by geriatric and other professional societies.10–13 Notably, the recent publication of updated versions of the American Geriatrics Society Beers Criteria (AGS Beers Criteria), has informed healthcare providers with comprehensive lists of potentially inappropriate medications for older adults.11–13
In our healthcare organisation’s care of patients, both nortriptyline (structurally classified a tricyclic antidepressant, TCA) and paroxetine, (functionally classified as a selective serotonin reuptake inhibitor, SSRI) have been extensively used for the management of generalised anxiety disorders and depression (paroxetine) and neuropathic pain syndromes (nortriptyline). However, these drugs have been associated with anticholinergic properties that may produce multiple potential adverse drug effects in older adult patients, including blurred vision, constipation, urinary retention, dizziness, falls, fractures, syncope, confusion, delirium and with chronic exposure, dementia.14–16 Due to these potential risks, the US Centers for Medicare and Medicaid Services, Healthcare Effectiveness Data and Information Set and other guidelines were established to advance quality pharmaceutical care in older adults. Consequently, large numbers of patients in our organisation who had obtained benefit from these medications underwent reassessment and, in many cases, were transitioned to other medications.
A review of the literature conducted by the investigators (search terms: Aged; Drug Therapy; Antidepressive Agents; Nortriptyline; Paroxetine; Cholinergic Antagonists; Drug-Related Side Effects and Adverse Reactions) did not reveal direct evidence clearly implicating these drugs as high-risk medications in older adult patients. We, therefore, conducted a retrospective cohort study in our large integrated healthcare organisation to compare the risk for adverse drug events associated with nortriptyline versus paroxetine and alternative medications prescribed for the treatment of depression, anxiety disorders and neuropathic pain in older adult patients.
Methods
Study population
The study was conducted at Kaiser Permanente Southern California (KPSC), a large integrated healthcare delivery system. KPSC has 4.8 million members across 15 medical centre areas in Southern California using a comprehensive electronic medical record and an integrated pharmacy prescription database.
The KPSC membership population reflects the sociodemographic diversity of Southern California.17 We report our findings according to The Strengthening the Reporting of Observational Studies in Epidemiology statement: guidelines for reporting observational studies.18
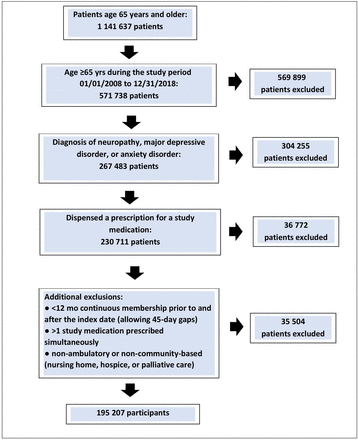
The population for the study were patients aged 65 years and older between 1 January 2008 and 31 December 2018, with a diagnosis of major depressive disorder, anxiety disorder or peripheral neuropathy and dispensed one of the study medications of interest: nortriptyline, amitriptyline, venlafaxine, duloxetine, fluoxetine, paroxetine, sertraline, citalopram, escitalopram or gabapentin. The diagnosis was based on documentation of International Classification of Diseases, 9th and 10 Revision codes (ICD-9 and ICD-10) entered at encounters with providers. The index date was defined as the date when a patient first met three criteria within the study period: 65 years of age or older, diagnosis of interest and prescription dispensed for a study medication. Patients were excluded if they met any of the following exclusion criteria: less than 12 months of continuous KPSC membership prior to and following the index date (allowing for 45-day gaps) and dispensed more than one study medication simultaneously. To obtain a clearer indication of adverse drug effects, frailer older adult patients with non-ambulatory status, nursing home residency, enrolment in hospice or palliative care were excluded. Patients were censored at the end of the study period, end of days-supply of the study prescription medication, meeting all three event outcomes, disenrollment from their health plan, or death, whichever occurred first. If a patient was subsequently prescribed and dispensed a different study medication, this generated a new index date and data were included if there was no overlap between the days-supply of two study medications. Dispensed prescriptions were identified based on the Generic Product Identifier code within the KPSC pharmacy database, the Enterprise Pharmacy Information Management System (figure 1).
Flow chart of study cohort. Participants could be included for a subsequent exposure to a different study medication if there was no overlap in days-supply between medications.
Study outcomes
The main study outcomes were falls, fractures and syncope defined by ICD-9 or ICD-10 codes recorded during outpatient, urgent care and emergency department encounters. Fractures were included regardless of cause or mechanism of injury; however, pathological fractures and vertebral osteoporotic compression fractures were not included in the study. We included outcomes if they occurred after the index date over an eleven-year study period, 1 January 2008 to 31 December 2018. Each type of outcome was only counted once. For example, after the first syncopal episode, no additional syncopal events were captured, but the patient would remain eligible for the capture of fall and fracture events. A fall or fracture outcome would only be included in the analysis if syncope was not coded within the same encounter.
Exposure
The exposures of interest were the 10 study medications identified above prescribed for the treatment of depression, anxiety disorder and peripheral neuropathy. All prescription data, including days-supply, were retrieved from the pharmacy electronic prescription database. A study medication episode was defined from index date until end of days-supply of dispensed prescriptions (including up to 100 days between dispenses), dispensing of a different study medication, outcome of interest (followed discretely for all three outcomes), end of study, disenrolment from health plan or death. A patient could be included for a subsequent episode with a different study medication if there was no overlap in days-supply between medications.
Covariates
The potential confounding variables included in the analysis were years of age at index date (65–74, 75–84 and ≥85 years), sex (female and male), race/ethnicity (white, Hispanic, black, Asian/Pacific Islanders and other), Charlson Comorbidity Score (0, 1–2 and ≥3), comorbidities within 12 months before the index date, history of falls at study entry (for the analysis of falls) and use of other drugs (sedating medications and anticholinergic medications) throughout the study.
Comorbidities that could potentially affect the study outcomes were ascertained at the index date using ICD-9 and ICD-10 codes; these include angina, acute myocardial infarction, congestive heart failure, cardiomyopathy, valvular heart disease, hypotension, adrenal insufficiency, hypoglycaemia, respiratory failure, alcohol abuse, visual impairment, gait disorder, arthritis, dementia, peripheral neuropathy, low bone density/osteoporosis, thyroid disease, malnutrition, Parkinson’s disease, cerebral palsy, cancer (excluding skin cancers), diabetes, epilepsy, cerebrovascular disease and unintentional weight loss. Each comorbidity was categorised as a binary variable.
Data on the use of certain additional medications were retrieved, including sedating drugs (opioids, barbiturates, benzodiazepines, non-benzodiazepine hypnotics) and medications with anticholinergic properties. A previously validated scoring of medications with anticholinergic properties, known as the Anticholinergic Cognitive Burden Scale (ACB scale) was used.15 The ACB score (categorised as 0, 1–2 and ≥3) and the number of sedating medications used (categorised as 0, 1, 2, ≥3) were time-dependent during the study period and were assessed whenever changes in these prescriptions occurred. This allowed each patient to have a daily value for these variables throughout the study.
Patient and public involvement
Patients and members of the public were not directly involved in the planning or conduct of this study.
Statistical analysis
Descriptive statistics were used to describe characteristics of the study cohort at baseline. Continuous variables are presented as means, median and range; categorical variables are presented in percentages. Cox’s proportional hazards regression models were used for separate analyses for each outcome. Time to first recorded diagnosis of each outcome was used as an outcome in each analysis. We treated study medication use as a time varying exposure to account for patients having more than one index date and medication episode. The dataset was summarised to evaluate patient-days and the crude incidence rates were computed as events per 100 patient-years.
The analysis calculated HRs with 95% CI for medication treatment, both unadjusted and adjusted for the confounding variables described above. This involved separate models of time dependent Cox proportional hazards regression for the study medications, sedating medications, anticholinergic medications, patient demographics, Charlson Comorbidity Score and finally one combined model. The main analyses examined each study medication compared with the reference medication, nortriptyline.
Following convention, a 95% CI that did not overlap 1.00 and a p<0.05 were considered statistically significant. SAS (V.9.4) was used for all analyses. Among patients who switched between multiple study medications, an outcome event that occurred during the exposure interval of one study medication could have been attributed to the previous study medication. To assess a potential spillover effect of one study medication onto another, a sensitivity analysis was performed for the sample of patients who only used one study medication during the study period. This sensitivity analysis will also assess possible channelling bias of medication selection.
Results
Study cohort and patient characteristics
A total of 195 207 patients in the KPSC electronic health record had a recorded diagnosis of depression, anxiety disorder, or peripheral neuropathy at the age of 65 and older between 1 January 2008 and 31 December 2018, and met all the inclusion/exclusion criteria (figure 1). The mean age of the final sample was 72.6 years, and the cohort contained 125 206 (64.1%) women and 70 001 (35.9%) men (table 1). The total number of patient-days on study medication was 81 767 400 patient-days, with a mean of 418.9 days per patient. Demographics and medical condition covariates differed slightly across study medications (online supplemental table S1) which is controlled for in the regression models.
Supplemental material
Baseline demographic and health characteristics of study participants
Patterns of study medication use
During follow-up, all patients were dispensed at least one prescription for a study medication, with a total of 1 192 787 prescriptions of study medication dispensed and a mean of 6.1 prescriptions dispensed per patient. A total of 169 830 (87.0% of 195 207) patients received prescriptions for a single study medication during the study period; 25 377 (13.0%) received two or more different study medications. Fluoxetine was the most dispensed medication, comprising 25.7% (176 743) of all study medication episodes, followed by nortriptyline at 16.6% (114 326) with escitalopram being the least prescribed at 1.9% (13 338). On average, the duration of a treatment episode was 113.4 days (range 85.4–140.3 days) (online supplemental table S2).
Absolute event rates overall and by study medication
The overall event rates for falls, fractures and syncope were 8.98, 6.72 and 4.87 events per 100-patient years, respectively. The combined event rates for the three outcomes for each study medication ranged from amitriptyline at 5.28 events per 100-patient years, to citalopram with 8.18 events per 100 patient-years; nortriptyline and paroxetine were 5.66 and 6.53 events per 100 patient-years, respectively (table 2).
Incidence rate (events/100 patients-years) of adverse outcomes by study medication
Association between study medications and adverse event outcomes
During the 11-year study period, 19 305 falls, 15 088 fractures and 11 313 episodes of syncope occurred (table 3). Compared with the reference medication, nortriptyline, the adjusted HRs (aHRs) for falls were statistically significantly greater for: amitriptyline (aHR 1.20, 95% CI 1.08 to 1.33), venlafaxine (1.44, 95% CI 1.34 to 1.56), duloxetine (1.25, 95% CI 1.12 to 1.40), fluoxetine (1.51, 95% CI 1.44 to 1.59), paroxetine (1.48, 95% CI 1.39 to 1.57), sertraline (1.53, 95% CI 1.44 to 1.62), citalopram (1.61, 95% CI 1.52 to 1.71) and escitalopram (1.37, 95% CI 1.21 to 1.54), but not gabapentin (0.95, 95% CI 0.89 to 1.02). For fractures, compared with nortriptyline, the aHRs were statistically significantly greater for: venlafaxine (1.48, 95% CI 1.36 to 1.61), duloxetine (1.47, 95% CI 1.32 to 1.64), fluoxetine (1.38, 95% CI 1.30 to 1.46), paroxetine (1.33, 95% CI 1.24 to 1.43), sertraline (1.43, 95% CI 1.34 to 1.53), citalopram (1.37, 95% CI 1.28 to 1.46), escitalopram (1.82, 95% CI 1.62 to 2.05) and gabapentin (1.30, 95% CI 1.22 to 1.39). For syncope, the aHRs were statistically significantly greater for: venlafaxine (1.21, 95% CI 1.10 to 1.34), fluoxetine (1.19, 95% CI 1.11 to 1.27), paroxetine (1.19, 95% CI 1.10 to 1.29), sertraline (1.30, 95% CI 1.20 to 1.40) and citalopram (1.30, 95% CI 1.21 to 1.39). In most comparisons, nortriptyline had a lower risk for adverse outcomes compared with the alternative study medications; paroxetine was similar in risk to the other study medications (table 3).
HRs for adverse outcomes by study medications in 195 207 participants, unadjusted and adjusted for confounders*
Association between patient characteristics and adverse event outcomes
Figure 2 and online supplemental table S3 present the association between patient characteristics (eg, age, sex, race/ethnicity, Charlson Comorbidity Score, use of sedating medications and the ACB score) and each of the three adverse outcomes. The aHRs increased with age: compared with the reference group of 65–74 years, the aHR (with 95% CI) was significantly greater for age band of 75–84 years: aHR for falls 1.98 (95% CI 1.92 to 2.04), fracture 1.32 (95% CI 1.28 to 1.37) and syncope 1.64 (95% CI 1.58 to 1.71) and higher again for age greater than 85 years: for falls 3.22 (95% CI 3.09 to 3.35), fracture 1.83 (95% CI 1.74 to 1.92) and syncope 2.10 (95% CI 1.99 to 2.22). Charlson Comorbidity Score also showed a positive association with the risks of all three outcomes: compared with the reference group of score 0, a score of 1–2 and score of ≥3 were associated with an increase in the aHR (with 95% CI) for falls of 1.10 (1.06 to 1.14) and 1.15 (1.10 to 1.20); for fractures 1.09 (1.05 to 1.14) and 1.08 (1.03 to 1.13) and for syncope 1.15 (1.10 to 1.21) and 1.32 (1.25 to 1.40). The use of sedating medications was significantly associated with elevated risks for all three adverse outcomes, especially for falls and fractures. Compared with non-users, the use of three or more sedating medications was associated with an increase in the aHRs (with 95% CI) for falls and fractures by 3.44 (2.97 to 3.99) and 4.41 (3.79 to 5.13), respectively. The concomitant use of multiple anticholinergic medications (ACB≥3) was also significantly associated with an increase in the risk of falls (aHR 1.37; 95% CI 1.32 to 1.42), syncope (1.31; 95% CI 1.24–1.38) and fractures (1.08; 95% CI 1.04 to 1.13) (online supplemental table S3).
Forest plots of adjusted HRs demonstrating association between adverse outcomes and: study medications, participant age, sex, race/ethnicity, Charlson Comorbidity Score; and exposure to sedating and anticholinergic medications. ACB, Anticholinergic Cognitive Burden.
Sensitivity analysis
Although most patients (87%) were exposed to only a single study medication during the study period, a small portion of patients switched between multiple study medications, raising concerns about whether an outcome event that occurred during the exposure interval of one study medication was in part due to the use of a previous study medication. To alleviate such a spillover effect between different study medications, a sensitivity analysis was conducted among those with only one study medication during the study period. The results (online supplemental table S4) were highly consistent with those derived from the analysis of all study patients (table 3).
Discussion
This retrospective cohort study of older adults with multiple comorbidities dispensed medication for depression, anxiety or neuropathic pain in a large integrated healthcare system found that nortriptyline represented a lower risk for adverse drug events than alternative medications for these indications. The tertiary-amine TCA amitriptyline, along with the SSRIs fluoxetine, sertraline, citalopram, and escitalopram, and two serotonin-norepinephrine reuptake inhibitors, venlafaxine and duloxetine, were generally associated with an increased risk for fall, fracture and syncope compared with nortriptyline, a secondary-amine TCA. The anticonvulsant gabapentin, an agent commonly prescribed for painful peripheral neuropathy, was found to represent a comparable risk to nortriptyline for syncope and falls, and an increased risk for fractures. Paroxetine was similar in risk for adverse outcomes to comparator antidepressants venlafaxine, duloxetine, fluoxetine, sertraline, citalopram and escitalopram. Statistical adjustment for a comprehensive list of comorbidities and confounding covariates did not alter these findings.
Strengths and limitations
This study was conducted using the electronic medical record and prescription drug database from a large, diverse integrated healthcare system consisting of 4.8 million members and 15 medical centre areas. The study medication cohorts were large, highly comparable and the duration of therapy substantial. Findings were adjusted in the Cox model based on multiple clinically relevant covariates. All eligible patients were included, including those with multiple comorbidities and concomitant medications, limiting selection bias and maximising external validity.
The identification of diagnoses and adverse drug events was dependent on ICD-9 and ICD-10 coding. There may be inaccuracies in initial coding by physicians; however, this would be expected to be similar across all ten medication groups. Only events that resulted in an injury with a healthcare system encounter were captured; this may underestimate the number of falls and syncope that occur while on these medications. While the duration of drug therapy was assessed, the drug dosage of nortriptyline was not directly examined. However, of the dosage forms of nortriptyline dispensed, 83% were the 25 mg capsules or lesser strength, and 98% were 50 mg capsules or lesser strength, suggesting treatment of neuropathic pain syndromes rather than major depressive disorder. Additionally, we note that utilisation of escitalopram was low. This may be attributable to its non-formulary status early in the study period: it was not added to the Drug Formulary until November 2012, 4 years 11 months into the study period. Subsequent utilisation may have remained low because candidates for this agent may have already been prescribed the closely related SSRI, citalopram. Drug dosing by thousands of different physicians in our healthcare system reflects the prevailing clinical use of these medications and may be generalisable to other clinical settings. The organisation emphasises evidence-based practice to reduce the use of inappropriate medications in older adults.
To minimise channelling bias, our analyses were adjusted for many clinically important comorbidities—including history of falls for the falls outcome group. We cannot exclude the possibility of other residual confounding on our results. A comparator group of subjects unexposed to a study medication was not included in the study design. This was a self-selected group of older patients desiring to be on medication for three different medical conditions and informs us of the relative risk between these medications. There could be potential bias in medication selection by doctors based on patient characteristics. This study addressed this issue by controlling for a comprehensive list of comorbidities that might have influenced doctors’ medication selection, but there might be other unobserved heterogeneity. We also allowed multiple medication episodes to be included to minimise this bias. The sensitivity analysis of patients who only received one study medication showed very similar results to the full analysis.
Comparisons with other studies
Supplementary evidence tables provided in the 2015 version of the AGS Beers Criteria12 cited three studies to support the inclusion of nortriptyline and paroxetine as potentially inappropriate medications in older adults19–21; however, these studies did not directly implicate these specific medications as having a higher risk for adverse drug events than comparative antidepressants not on the AGS/Beers Criteria.
These two medications, widely used in general clinical practice for the management of major depressive disorders and generalised anxiety disorders (paroxetine), and for painful peripheral neuropathies (nortriptyline), have been the subject of numerous studies on the safety and efficacy of pharmacotherapy in older adults.
Coupland et al19 found SSRIs used for the treatment of depression in adults aged 65 years and older had a significantly increased risk for several adverse drug events. The aHR for falls for SSRIs was 1.66 (95% CI 1.58 to 1.73) compared with the reference group of the same patients when not taking antidepressants. For fracture the aHR for SSRIs was 1.58 (95% CI 1.48 to 1.68). The same study also showed that TCAs had a significantly increased risk of falls and fracture compared with the reference group, but the risk was less than for SSRIs. Our findings were in general agreement with this, with SSRIs demonstrating greater aHRs than the secondary-amine TCA nortriptyline. Our study demonstrated a substantially greater difference between nortriptyline and SSRIs, with the risk of falls ranging from 36.6% to 61.3% higher in the SSRI group compared with nortriptyline. Several other studies assessed risk for falls, where SSRI antidepressants were found to strongly correlate with falls.22–24 Additional studies found that both TCAs and SSRIs were associated with increased fracture rates, with potentially higher rates with SSRIs.25–27 These risks seemed sustained across multiple observational studies.
In a review of 78 studies of TCAs and SSRI antidepressants, Darowski et al28 found the risk for falls attributable to specific antidepressants to be similar and comparable across studies.
Gagne et al29 examined fracture rates among propensity score-matched cohorts in a MediCare patient population on antidepressant treatment and found that, compared with secondary-amine TCAs (eg, nortriptyline), SSRIs had the highest association with fracture rates (HR 1.30, 95% CI 1.12 to 1.52), compared with tertiary-TCAs (eg, amitriptyline) (HR 1.01, 95% CI 0.87 to 1.18) and atypical antidepressants (1.12, 95% CI 0.96 to 1.31). More recently, using MediCare data, Bali et al30 conducted a propensity score-matched retrospective cohort study of 4620 elderly nursing home patients prescribed SSRI antidepressants for depression and found no significant differences in mortality between paroxetine and other SSRIs (aHR 1.01, 95% CI 0.86 to 1.19).
Implications and future research
The results of this observational cohort study suggest that nortriptyline and paroxetine could be considered appropriate therapy in older adult patients with diagnoses of generalised anxiety disorder, major depressive disorder, painful neuropathic pain along with multiple comorbidities in older adults, when medication is indicated and desired. Future research should use a prospective, randomised controlled study design to further address questions of risk for major adverse events and to confirm or refute the safety of these medications in older adults.
Conclusions
In this large retrospective cohort study, nortriptyline was found to represent a lower risk for falls, fractures and syncope versus comparator medications; paroxetine was comparable to these alternative medications. Findings from our large retrospective cohort study support the use of nortriptyline or paroxetine for appropriate indications in older adult patients.
Data availability statement
No data are available. The data underlying this article are not shared due to the privacy policy of data providers.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and the Kaiser Permanente Southern California Institutional Review Board approved this study with waiver of informed consent (approval no. 11554). This is a data review study that does not require any direct contact with the study subjects. The research involves no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required outside of the research context.
Acknowledgments
Jiaxiao M Shi, PhD and Raoul J Burchette, MS provided input on statistical methods and early statistical analysis. Stephanie Tovar, MS provided support as our project manager. Cristella Torres, MD and Nichole Koshki, PharmD were involved in the literature review, meetings and poster creation and presentation.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors All authors contributed to the study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content. MMG and RLD developed the study design. DD completed the statistical analysis. MMG and RLD drafted the manuscript. MMG and RLD are guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding This research was supported by a grant from the Regional Research Committee of Kaiser Permanente Southern California, RRC grant number: KP-RRC-20181106.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.