Article Text
Abstract
Background The Improving the Wellbeing of people with Opioid Treated CHronic pain (I-WOTCH) randomised controlled trial found that a group-based educational intervention to support people using strong opioids for chronic non-malignant pain helped a significant proportion of people to stop or decrease opioid use with no increase in pain-related disability. We report a linked process evaluation of the group-based intervention evaluated in comparison to a usual-care control group that received a self-help booklet and relaxation CD.
Methods We interviewed 18 intervention facilitators, and 20 intervention and 20 control participants who had chronic non-malignant pain and were recruited from general (family) practices in the UK. Quantitative data included change mechanism questions on the trial questionnaires which explored motivation, expectations and self-efficacy. Fidelity was assessed by listening to a sample of audio-recorded group sessions and nurse consultations. Quantitative and qualitative data were integrated using ‘follow a thread’ and a mixed-methods matrix.
Findings Four overarching themes emerged: (1) the right time to taper, (2) the backdrop of a life with chronic pain, (3) needing support and (4) the benefits of being in a group. Delivery fidelity was good, adherence (83%) and competence (79%) across a range of intervention groups. Staff delivering the intervention found three typical responses to the intervention: resistance, open to trying and feeling it was not the right time. The group experience was important to those in the intervention arm. It provided people with a forum in which to learn about the current thinking about opioid usage and its effects. It also gave them examples of how feasible or personally relevant coming off opioids might be.
Conclusion The process evaluation data showed that the I-WOTCH intervention was well delivered, well received and useful for most interviewees. Being ‘the right time’ to taper and having support throughout tapering, emerged as important factors within the context of living with chronic pain.
Trial registration number ISRCTN49470934.
- Opioid
- process evaluation
- chronic non-malignant pain
- pain self management
- tapering
- qualitative
- behaviour change
- complex interventions
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
- Opioid
- process evaluation
- chronic non-malignant pain
- pain self management
- tapering
- qualitative
- behaviour change
- complex interventions
STRENGTHS AND LIMITATIONS OF THIS STUDY
The success of I-WOTCH was demonstrated from multiple perspectives providing an insight into the acceptability, replicability and transferability of the intervention.
Enablers of, and barriers to, the tapering process were explored, both of which are valuable for tailoring of this type of intervention to the individual.
This protocol-based process evaluation showed good fidelity of delivery, at least minimal compliance in 62%, and a very positive experience of the intervention, suggesting its suitability for service rollout.
Although general practitioners screened patients and prescribed for tapering, their perspectives were not a part of this process evaluation but may have provided useful information for future implementation.
As 95% of participants in the process evaluation were white, the reach of the study was thus limited and given interviews were 12 months after the intervention, recall bias may be a limitation.
Background
Embedded process evaluations of complex interventions in randomised controlled trials (RCTs) are critical to increase understanding of how and why interventions may or may not work, as well as how practical interventions are when implemented in service contexts. They increase confidence in trial results and provide guidance on their implementation.1 2
In the landmark Improving the Wellbeing of people with Opioid Treated CHronic pain (I-WOTCH) trial (n=608), we demonstrated that a self-management support intervention helps people using strong opioids become opioid-free at 1 year: 29% intervention versus 7% control, OR 6.55 (95% CI 3.42 to 12.55) with no increase in pain interference.
The I-WOTCH intervention included educational, behavioural and psychological components, combining group and one-to-one support. Papers reporting the intervention development, the trial protocol, the main results and process evaluation protocol have been published.3–6 Here we report the I-WOTCH process evaluation, specifically.
Experiences of the I-WOTCH intervention(s): including enablers of, and barriers to, change among participants.
Implementation of the I-WOTCH group intervention: exploring the dose delivered and received, and the fidelity of delivery.
Change mechanisms potentially underpinning intervention effects.
Contextual issues: exploring how these may affect the outcome or running of the study and/or intervention.
Methods
We explored experiences of the participants and intervention delivery staff as well as key areas of process evaluation: context (contextual factors which may affect the implementation), fidelity (whether the intervention was delivered as conceived), dose delivered (the amount of the intervention delivered) and dose received (the amount of the intervention received by participants).7 The group sessions took place once a week for 3 weeks. There was a face-to-face consultation between the second and third group sessions. After the third group session, there were two telephone conversations and then a final face-to-face consultation over a total period of 9–10 weeks. The process evaluation ran alongside the main study from May 2017 to March 2020.
The I-WOTCH trial recruited participants with chronic non-malignant pain from general (family) practices from midlands and northeast of England (table 1). Inclusion and exclusion criteria are listed in box 1. Once randomised all participants received a self-help booklet ‘My Opioid Manager’ and a relaxation CD. The intervention group also received a 3-day group intervention, run by a specially trained nurse/other HCPs and lay person with chronic pain who had tapered their own opioids, with two additional face-to-face nurse consultations and telephone support.
I-WOTCH participant inclusion and exclusion criteria
Inclusion criteria
Provision of written informed consent.
Aged 18 years old or above.
Using opioids for chronic non-malignant pain.
Using strong opioids for at least 3 months.
Using strong opioids on most days in the preceding month.
Fluent in written and spoken English.
Able to attend group sessions.
Willing for GP to be informed of participation.
Exclusion criteria
Regular use of injected opioid drugs.
Chronic headache as the dominant painful disorder.
Serious mental health problems that preclude participation in a group intervention.
Previous entry or randomisation in the present trial.
Participation in a clinical trial of an investigational medicinal product in the last 90 days.
Pregnant at time of eligibility assessment, or actively trying to become pregnant.
People receiving strong opioid for the management of pain due to active malignant disease.
I-WOTCH process evaluation: aims, key components, methods, analyses and data type
We used process evaluation theory to devise a logic model specific to this intervention and its theoretical underpinnings, informed by The Information, Motivation and Behaviour Skills Model8 9 which highlights that knowledge, strong and stable motivation, and prerequisite skills are needed to initiate and maintain behaviour change (online supplemental material 1—logic model). We used a mixed-methods approach for the process evaluation (table 1).
Supplemental material
Interviews
Potential interviewees (participants and staff) were sent an information leaflet. Those interested gave informed consent to be interviewed. Intervention delivery staff were told of the interviews at their training and were approached on completing their final group. Semistructured interviews were recorded using an encrypted device (OLYMPUS DS-7000 digital voice recorder), see online supplemental material 2 for indicative topic guide. Recordings were transcribed verbatim, anonymised, given numerical identifiers to maintain confidentiality and checked for accuracy by the research interviewer (VPN). The data were analysed using NVivo V.12 software to explore and organise the data. Warwick CTU’s (clinical trials unit) lone worker policy, including a risk assessment, was followed for interview visits.
All data were collected and stored in digitally secure locations with restricted access in accordance with the Data Protection Act 2018c12 which is the UK’s implementation of the General Data Protection Regulation.10
Participant feedback forms
We gave feedback forms to the last 10 groups of participants after completing day 3 of the intervention. Questions asked about different aspects including how the group intervention was delivered and what they had found personally useful/not useful.
Dose delivered and dose received
We collated trial data including date, location and attendance of the intervention groups to determine the dose delivered and received.
Fidelity
Our fidelity assessment is detailed in our protocol paper and online supplemental material 3 shows our random sampling.5 We listened to audio recordings of 11 prespecified, intervention group sessions across days 1, 2 and 3 (those which were educational and promoted discussion see online supplemental material 4) to assess adherence to the manual and competence of delivery. Other more practical sessions were checked for content presence but not rated in terms of delivery competence. We used bespoke assessment forms for group and individual sessions across a range of facilitators. Adherence items were scored as taking place: yes (2), partially (1) or no (0). Competence items were scored as: evident (2), partially evident (1) or not evident (0) (see online supplemental material 5). Scores were summated and averages calculated for adherence and competence of each session.
A random 10% of first and second one-to-one nurse/patient consultation recordings were also assessed throughout the study. If audio recordings were missing or inaudible, then the same sessions as those missing would be taken from the next group or one-to-one consultation in the same area (northeast or midlands). A second researcher (KS) double coded 10% of all the assessments.
Potential change mechanisms
Four change mechanism questions about: patient motivations, expectations, self-efficacy and perceived credibility of the intervention were added to the main trial questionnaire to ascertain if any of these factors had an effect on opioid reduction (online supplemental material 6).
The process evaluation team
The process evaluation team included: KS, a co-applicant and work package lead who has a long track record in qualitative and mixed-methods research in complex interventions, CA, a co-applicant, highly experienced in behaviour change research and process evaluations and VPN, a research fellow with a background in physiotherapy, experienced in mixed-methods in rehabilitation RCTs. The team collated data and analysed it separately from the main study. The initial report was given to the senior and chief investigators SE, HKS and MU prior to the results from the main trial being available.
Patient and public involvement statement
Patient and public involvement input was not used directly with this process evaluation although one lay advisor, recruited via UNTRAP (Universities/User Teaching and Research Action Partnership) who helped with the development of I-WOTCH and its intervention, stayed on to become a valuable team member. Study findings will be disseminated via newsletters and a lay summary put onto the study website as well as feedback to UNTRAP and other partnerships as outlined in the I-WOTCH protocol paper.5
Findings
Experiences of the I-WOTCH interventions
We had multiple sources of qualitative data (figure 1).
Qualitative data sources. I-WOTCH, Improving the Wellbeing of people with Opioid Treated CHronic pain.
Participant interviews
The 40 interviewees (20 control/20 intervention) had a mean age of 65, range 59–72 years, 38/40 (95%) were white and 25 (63%) were women. Half (50%) had been on opioids for >5 years and 31 (78%) had pain for >5 years. See online supplemental materials 7 and 8 for full interviewee characteristics and compared with the main study demographics, noting the interview samples were broadly consistent with the main study. Eighteen participants declined an interview mostly because of ‘health issues’ (eg, appointments or operations) or ‘not a good time’ (eg, work commitments, too busy).
Each interviewee has been given a numerical identifier, which follows each presented quotation. Table 2 provides evidence from the control interviews.
Control exemplar quotes
Control interviews
The majority (14/20) found the self-help booklet informative, useful for further resources they could access. Three did not remember receiving the self-learning manual and two found some of it difficult to understand.
Most (n=10) listened to the CD once or twice and did not find it useful. Another four who used it more than once or twice said that ultimately it was not useful for them. However, five participants used it to good effect over the trial period, some on a regular basis and others ‘as and when’ they felt they needed it.
Enablers and barriers to tapering
In the interview schedule, we asked all interviewees about enablers and barriers to tapering. We analysed the data from all 40 patient interviews using framework analysis for enablers and barriers to the tapering process including any attribution to the I-WOTCH study from the participants’ perspectives.
As we are focussing on the I-WOTCH intervention for this paper, we illustrate the themes with quotations from the intervention arm only for clarity.
Enablers
We identified four themes: (1) readiness to start tapering, (2) I-WOTCH as a trigger or motivator, (3) continuing to taper (once they had started) and (4) living without opioids. Subthemes are listed in table 3 with exemplar quotes from the intervention participants.
Enablers to tapering opioids
Barriers to tapering
Three barrier themes are from different phases of tapering: pre-tapering, during tapering and after stopping opioids. Subthemes are given in table 4A–C with exemplar quotes from the intervention.
Barriers to tapering
The role of the general practitioner (GP)
Most interviewees spoke about their GP in relation to their opioid use and tapering and the role of GP is presented in table 5. In the I-WOTCH study, intervention participants were given a tapering plan which was also sent to their GP and people were encouraged to make an appointment and discuss the plan. It was the GP who then actioned the plan if acceptable to both parties. The self-help booklet also suggested control participants could discuss tapering with their GPs. We have thus used quotations from intervention and control groups for this section on the role of the GP. There were five themes: (1) GP as information giver and sounding board, (2) trust in doctor, (3) teamwork, (4) GP reluctance and (5) access to GP. Table 5 gives exemplar quotes.
Role of the GP
Intervention interviews—participants’ experiences of the intervention (n=20)
We present four main themes with subthemes where appropriate.
Acceptability of the intervention.
Being in a group.
Shared experience.
Social comparisons.
Support.
Being committed to something.
Group numbers.
Perceptions about group facilitators.
Challenges to establishing group cohesion.
Group sessions of interest.
Opioid information.
Distraction techniques.
Anger irritability and frustration.
Relaxation.
Mindfulness.
Nurse one-to-one consultations: participant perspectives.
Acceptability of the intervention
Half said that the intervention was delivered in an appropriate format. When asked about suggestions to improve it, key responses were: provision of more support especially as tapering progressed, more group time, less didactic delivery and more local venues. All agreed they would recommend the I-WOTCH approach to others although some noted that participants needed to be open and receptive.
Being in a group
Looking back on the group intervention, most interviewees found that being in a group was beneficial. They spoke about the groups as a shared experience where they were with people in similar circumstances which included the lay facilitator with whom they could share experiences. There were social comparisons within the group with participants noticing that others were like, or not like, themselves. Social support from the group was a key theme with evidence of people encouraging others especially when they were experiencing difficulties. Interviewees often talked about the commitment to the group and or the trial. Group numbers seemed important, less than three members limited discussion. Lay group facilitators were seen either as good examples of how people cope with chronic pain or, alternatively, that they were somehow not the same and may not have had the same long-term experience of opioids and pain. The former perception allowing greater identification with the lay facilitator. Not all groups ran smoothly especially if they included disruptive individuals (figure 2).
Being in a group.
Group session aspects of interest
There were five sessions which people talked about repeatedly across the data (see box 2) but on the whole people tended to talk globally about the intervention even when prompted to discuss individual sessions.
Specific aspects spoken about repeatedly.
Aspects of interest
Opioid information:
Information sessions gave most participants new insights into opioids and their effects. Participants also learnt about differences between dependence, tolerance and addiction and the effects of withdrawal and/or tapering and valued exploring these in the groups.
Distraction techniques:
Distraction techniques were felt to be the most useful technique, some recognised the technique in existing pastimes and hobbies.
Anger irritability and frustration:
This session was appreciated because participants felt they were not alone in having these feelings and that they had an opportunity to talk about them.
Relaxation:
Most liked the relaxation sessions but few used relaxation techniques regularly, as needed. Participants often confused relaxation with mindfulness.
Mindfulness:
Unless exposed to this previously, this was not well understood and often thought to be another form of relaxation. A few participants remained unsure about what it was.
Nurse one-to-one consultations: participant perspective
Most intervention participants talked about these sessions. A majority appreciated the tailoring of the tapering plan and how they could personally apply the elements they had learnt from the intervention. Sessions were described as encouraging and supportive and almost all talked about having a good relationship with the nurse.
… she was hopeful she said I had the determination to succeed in coming off the Tramadol… erm… so I felt positive with that… that I had like sort of the support with the words that I could actually do it … … I just felt encouraged like that I could do it meself so I liked the support I suppose and the fact that they believed in me…yeah! 13
Participant feedback forms
Participants were given feedback forms at the end of day 3 in a subset of 10 intervention groups, providing 31 responses (delays in submitting an amendment about this for ethical approval meant the earlier groups were not able to be asked to complete this feedback form). Overall, responses indicate positive appreciation of most aspects of the course, but this is a small subsample, reported in detail in online supplemental material 9 and should be treated with caution.
Intervention delivery staff interviews
Staff characteristics
We interviewed 18 intervention-delivery staff; nurse facilitators n=10, lay facilitators n=6 and other healthcare professional (HCP) facilitators n=2. Four (one nurse, three lay) did not respond. Identifier prefixes after each quotation are N for nurses, L for lay and H for HCPs.
We have presented the four themes from the staff interviews: (1) acceptability and training, (2) running the group sessions (venues, facilitators and group dynamics), (3) factors affecting readiness to change across group sessions and (4) one-to-one sessions.
Acceptability and training
Almost all were happy with the package as delivered. Quality assurance assessment on day 1 was appreciated as was the general support from the team ‘I wanted that there was somebody there on the first day actually because it was quite good to find out how… whether I was actually doing it [correctly]…’ L05
Staff interviewees regarded the training as adequate and the manual as comprehensive.
The manual ‘was very good because it took you through every single section very, very clearly.’ (N09)
‘….it’s quite thorough and really, really detailed.’ L03
Some would have liked more training on; practical facilitation and/or subjects with which they were not familiar, for example, mindfulness or teaching posture. Most put in extra unpaid time to read around relevant subjects and to practice their delivery, and most were happy doing this.
Running the sessions
Venues varied greatly, particularly in community settings. There were instances when even working to a lone worker risk-assessment protocol, facilitators were asked to lock up, being last in the building. On a few occasions, telephone back up with the I-WOTCH team was compromised by a lack of signal. IT problems were mitigated by facilitators having written scripts as backup.
Facilitators mostly worked well together, with only occasional disagreements over differences in delivery or choice of sessions. Most would have preferred to work in the same pairings which was not possible within the intervention delivery design. Nurses felt the days were quite ‘full on’ having little downtime because even in breaks and meal-breaks everybody still chatted. Lay facilitators who were having to deal with their own health issues commented that the travel and full days (09:00 to 15:00) left them tired. However, all the facilitators said that the lay facilitator role was key to the groups running well. ‘… do not remove the lay facilitators if you’re going forward, they have to be there…’ N02
Group dynamics
There was sometimes initial scepticism and negativity about the programme which was challenging for the staff to deal with. Resistance sometimes changed around day 2.
… I think by sort of early afternoon is when people started you know listening a bit more thinking, ‘Oh ok maybe there is an alternative?’ L07
Groups fared differently—occasionally groups bonded or ‘gelled’ as early as day 1 but more often by the end of day 2 and definitely by day 3 where shared experiences and supportive bonds had been formed.
Now dealing with positive people looking and moving forward. L04
The most challenging groups involved a small minority of participants actively resistant to the information and techniques offered. Some showed challenging behaviour such as ‘rubbishing’ the content or interrupting. Some of these participants did not return after day 1.
‘Shutters down’ straight away. L05
Factors affecting readiness to change across group sessions—categories of change
Intervention delivery staff described three presentations of change experience (across participants) and two turning points around the two main opioid information sessions. See boxes 3,4.
Three categories of change experience identified by intervention delivery staff
Resistance to change
Some did not want to engage, either that they were sceptical about the information, had no side effects or were fearful of coming off opioids. This was sometimes due to bad experiences or felt reliant on them or wanted something else as a substitute.
‘… that was the bit they all dreaded… mmm… and they were super nervous and a lot of them I think weren’t quite sure whether they wanted to take that step…’ L05
‘some people seemed to have no intention of looking at other ways to deal with their pain. Not Ready. L04. People ‘start off pessimistic’’ L05
Open to trying
Some participants were ready to try—motivated themselves or by the group or that they felt the medication perhaps wasn’t working anyway. There was concern after learning more about opioids especially its effects, tolerance and dependence. Tapering slowly was a reassuring message rather than ‘all or nothing’ enabling participants to ‘just give it a go.’
‘and sometimes they would talk themselves into it and talk themselves out of it… it was… cos it’s a step into the unknown and we tell them this is what could possibly affect you …it did make it worse I… … cos some of the patients felt that they were already dealing with quite a bit you know to start with…… what made it really good was…… the other facilitator the nurse said you know ‘it need not be a dramatic drop it can be a gentle tapering thing’ and I think when they saw the tapering that settled them down at the end…’ L05
‘Became friends…Reassurance that they are not alone’ L07
Not the right time
Staff spoke about participants wanting to delay tapering until after an event: for example, Holiday or surgery. They felt some were not able to manage it at that time but could reduce a little and try again later.
‘… there was only the one man at first on the first class who hadn’t been on opioids long… didn’t feel dependent on them yet but he said he’d learnt and he knew what to look out for so yes he would be you know aiming to come off them but not quite yet…’ L04
Turning points identified by intervention delivery staff
1) On the morning of day 1 some were shocked and unaware of the issues and surprised to learn opioids aren’t helpful long term.
Bit of an eye opener N09
‘I think they were surprised to learn that opioids aren’t helpful long term… … they would’ve been I think of the view that… err… I need to take this because if I don’t take it I’ll be in even more pain rather than this doesn’t seem to be working cos I’m still in pain after 20 years!’ N11
‘(Angry with Doctors] and I think they were quite cross that they were getting it and being giving it and the length of time they’d been given… mmm that didn’t go down too well in any of the groups!’ L05
2) On the morning of day 2 when they started to think how they may start tapering and asked more questions. This was after the second session about opioids which covered withdrawal and John’s tapering story which worked well.
‘…my opinion was that they really needed their hands holding if they were going to make the jump…’ H2
‘… and then on day two pretty much you can tell who is more open to it ……most of the time it was the majority of the group you’d have the one or two that were still sceptical during day two still kind of like asking us questions…’ L03
One-to-one sessions: nurse facilitator perspective
Nurse facilitators spoke of the tapering app being straightforward to use, although at times they were unable to get a mobile phone signal and occasionally the opioid the patient was taking was not included in the tapering app. Hard copies of the tapering plan were also written. On discussing the tapering plans suggested on the app algorithm, nurses and participants often wanted to taper more slowly for example, tramadol tablets decreasing by 50mgs but with no smaller dosages available, lack of confidence or having withdrawal effects. This was supported and nurses felt that it was important that the participant felt in control of the process.
…a lot of the participants … mmm… didn’t feel that they could taper at that speed and my sort of take on that is that it is a marathon not a sprint and you know if you can’t taper at that speed then you know we’ll go a bit further …’ N10
Nurses described participants wanting to talk about a lot of issues which they hadn’t necessarily brought to the group and that they needed that opportunity to talk.
it gives them an opportunity to talk openly with me and perhaps mention things that they haven’t been able to mention within the group. N10
Some nurses spoke about seeing a difference in participants at their first and second face to face.
…people used phrases like they’d felt they’d ‘come from behind a curtain.’ And they didn’t seem quite so dazed… N06
Nurse data suggested that confidence, motivation and the participant being in control were key factors to consider in the tapering process.
Implementation of the I-WOTCH group intervention
This section explores how the trial was implemented and the uptake of the components offered (figure 3).
Structure of the implementation items. I-WOTCH, Improving the Wellbeing of people with Opioid Treated CHronic pain.
Groups run (dose delivered)
The trial ran 35 groups—20 in the midlands, 15 in the northeast, of England. At randomisation, the mean group size was 9, SD 2.9, median group size 9 and IQR 5–11
Uptake and attendance (dose received)
Minimal compliance is defined as attending at least day 1 and the first one face-to-face consultation. There were 190 (62%) with at least minimal compliance. Full compliance is defined as attending at least days 1, 2 and 3, the first one-to-one consultation and at least one phone call. There were 144 participants with full compliance (47%).
We randomised 305 people to the group intervention with 166 (54%) attending all three group days. The first one-to-one session attendance was 190 (62%) and 131 (43%) attended both the first and second session. One hundred and sixty-seven (55%) received one or more telephone calls. Thirteen (4%) did not return after day one (as mentioned in the staff interviews).
Non-attendance at the first and subsequent sessions was high at 90 (30%) (full trial attendance see online supplemental material 10).
Participant reasons for non-attendance throughout the study
Reasons for non-attendance (if given) were poor health, competing work commitments and family interests.
Fidelity
Fidelity score averages for the group sessions adherence was 83% (very good 81%–90% as rated by Borrelli et al11), with a range of 25–100 and a median of 88 and overall competence 79% (good 71%–80%), range 0–100, median 86.11 One-to-one session scores were high with an adherence average percentage score of 91%, range 61–100 and competence an average of 93%, range 50–100 (excellent 91%–100%11) (online supplemental materials 11 and 12). The 25% adherence score was rated when there was a technical problem and the facilitator did not use the back-up scripts provided, so did not cover that section of the session as expected. The lack of facilitation skills denoted by a score of 0 on competence does suggest facilitation is a skill shared by most but not by all.
Potential change mechanisms
The control and intervention scores are comparable over the items of each question at baseline.
The two baseline only questions (2 and 3) (online supplemental material 6) were: Baseline expectation: I expect that, in 4 months’ time, I will have reduced my opioid use and Baseline self-efficacy I am confident I could reduce my opioid use a lot over 4 months which showed a trend of low expectations of successful tapering within 4 months and low self-efficacy/confidence in tapering (online supplemental material 13).
The motivation to reduce opioids question showed a trend for more participants wanting to cut down or stop their opioids at baseline. There is a weak trend showing the self-management group to be more slightly more motivated to continue tapering at later timepoints. However, this should be viewed with caution due to the high proportion of missing data for this item.
Our statistician ran statistical analyses on the full data collected; baseline 4, 8 and 12 months to look for any impact of responses to the change mechanism questions on main trial outcomes (see online supplemental material 14). There were few significant correlations except for the study perceived credibility question. Correlations indicate that more participants attributed their tapering success to involvement in the intervention arm of I-WOTCH with the reverse trend in the control group (online supplemental material 15).
Contextual issues
The main contextual issues were trying to live a life, often with multiple health problems and medications alongside managing multiple appointments, tests and operations. Day-to-day life is often a challenge dealing with their pain and the things they need to get done. Living with chronic pain can be challenging physically, mentally and emotionally. For some just the idea of starting to taper is ‘a step too far’ that they may be unwilling or unable to consider. This was seen in the participant interviews and the staff interviews especially with the three categories of change given previously in box 3 when people are: ‘Resistant to change’, ‘Open to trying’ or that it’s ‘Not the right time’.
Overarching themes from integration of qualitative and quantitative data
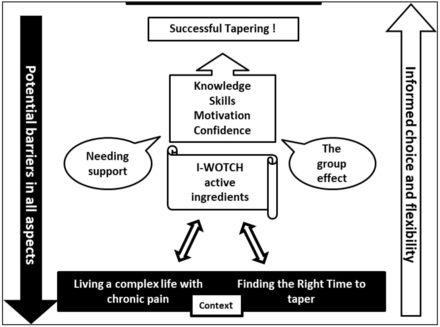
Analyses revealed threads across the qualitative and quantitative data which generated four main overarching themes: (1) the right time, (2) the backdrop of life with chronic pain, (3) needing support and (4) the group effect.
The right time. This theme encompasses evidence indicating whether someone is ready to change, including enablers or barriers to tapering and whether participants felt fully informed, motivated and confident to be able to taper.
Life with chronic pain is often complex with many having multiple health problems and medications. This means that participants were living life around their pain, pain relief, healthcare appointments and procedures as well as social and family commitments. Each of these could affect their decision about when or whether to taper.
Support is needed at all phases of tapering. This could come from their family, GP or from a tapering intervention such as I-WOTCH which agreed a tailored tapering programme. If family members or their GPs were ambivalent or against tapering, people were less keen to try it.
The group effect allowed for people taking opioid medication to come together to gain more information and skills in pain management as well as choosing whether to start on a tapering path as a joint group goal. They were given the opportunity to discuss and explore their fears and motivations about decreasing their medication.
The interaction between the overarching themes can be seen in our model. See figure 4. Participants make decisions about tapering based on whether it is the right time for them, their current pain experiences and healthcare and the support available to them. Helping participants to identify and discuss potential barriers while fostering knowledge, motivation, confidence and self-management skills means that tapering is more likely to take place.
Conceptual model of overarching themes. I-WOTCH, Improving the Wellbeing of people with Opioid Treated CHronic pain.
Discussion
The I-WOTCH main trial results showed that 62/225 (29%) of participants in the intervention group stopped taking opioids, compared with 15/208 (7%) in the usual-care group at 12 month follow-up without any difference in pain, or pain related disability, between the study arms. The process evaluation data indicate that the intervention was delivered as intended with good fidelity scores. There was evidence of participants attributing their opioid reduction to the I-WOTCH study. Participants and staff alike felt the package as whole was acceptable and deliverable. However, poor attendance was an issue with 90 participants randomised to the intervention not attending any group.
The secret ingredient?
No one factor or ‘secret ingredient’ was identified but rather a combination of active ingredients as enablers of opioid tapering. The I-WOTCH intervention seemed to have a synergistic, summative effect from its different components which could be specific to the individual. The qualitative data indicate that the intervention served to inform and provide a forum where discussion in groups and in one-to-one sessions could help people decide whether to reduce their opioid use. The final decision was the participant’s, and no judgements were made about whether or when they might start tapering.
The I-WOTCH intervention provided a space in which people could fill gaps in their knowledge of opioids. The intervention also enhanced motivations to taper, including intrinsic motivations that participants brought to the sessions. Information provided by the intervention about the lack of long-term effectiveness and side effects developed motivation. This was emphasised in staff interviews which highlighted how provision of information about opioid use consequences was, for some participants, a turning point in becoming motivated to change. Support from their GP, their family, the I-WOTCH one-to-one meetings, the tapering plan and group peers also enhanced motivation. Exploring pain management skills in a group over three sessions allowed them to try things out and swap ideas, so establishing the skill base needed for change.
The Information, Motivation and Behavioural Skills model8 9 provided the theoretical foundation and guided our understanding of key components of change that would support action towards opioid tapering. The qualitative data confirm the utility of identifying sources of information, developing motivation at the right time and skill development, including use of the lay facilitator as a role model, as critical precursors of change. This process evaluation reveals how the I-WOTCH intervention provided all three of these critical supports. Barriers to change may be specific to the individual but supporting people to become informed and to develop motivation and skills optimises the chances of behaviour change. The main barriers were that it might not have been the right time for participants to consider tapering and other things in life often took priority. Fears of returning to uncontrolled pain were common alongside multiple other potential barriers.
These findings correspond to a wider literature. A 2020 qualitative evidence synthesis using meta-ethnography (31 studies) looking at experiences of people taking opioids for chronic non-malignant pain by the current authors,12 found five themes, four of which resonated with our findings. The fifth theme was around societal stigma, which did not emerge from the data in this English study but was common in North American studies, where law enforcement agencies can be involved.13
Our findings are also broadly supported by recent literature. Goesling et al who held focus groups with former opioid users about their experiences before, during and after opioid cessation.14 Motivators to stop included concerns about a lack of opioid efficacy, addiction and quality of life impact. Quinlan et al noted that 60 patients undertaking a pre tapering survey found there were more barriers given than motivators.15 The main areas of concern were around quality of life, pain and withdrawal which the authors suggest need to be addressed for successful tapering to take place. Henry et al conducted focus groups and interviews with 21 adults with chronic pain at different stages of opioid tapering.16 They explored the complex contextual backdrop of life with chronic pain describing changes or fluctuations in many areas including pain, social relationships, health status, emotional state and the perceived need for opioids at any given time. They recognise the substantial effort involved for people undertaking the tapering journey, the effect on their day to day lives and the strategies used. They suggest early anticipatory guidance about tapering and that tapering should be patient centred and responsive to the patients’ needs.
We identified GPs as integral to tapering support. Three 2020–2022 papers from healthcare providers perspective17–19 throw light on the complex challenges involved in tapering opioids, especially the importance of maintaining a good patient/provider relationship. Hamilton et al also cite patient motivation to be a central factor for successful tapering.19 GPs are seen as authoritative experts in relation to health behaviour and patients often welcome behaviour change advice from their GPs, especially when such advice may have a positive effect on long-term condition management.20
There is a long history of successful use of small groups to develop support, share common health problems, build trust, share ideas, enhance information, change attitudes and develop motivation and skills to promote health behaviour change.21 22 This evaluation shows that the I-WOTCH intervention successfully applied these group processes in supporting individual change. Participants often had a strong sense of shared experience around opioid use and the challenges of use reduction. They felt as if they were all in the same boat. This is a key foundation for successful group interventions.23 There is also evidence of participants experiencing social support and social learning both key to individual change. Facilitation of interaction in small groups is critical to optimising change and interviews revealed that the lay facilitator role was very important to participants and nursing facilitators alike. Facilitators did not underestimate the challenges some participants might face and appreciated the lay facilitator’s personal experience with which participants could identity and then learn from. The challenges of group facilitation observed also emphasise the importance of careful skill-based training for group facilitators.22 At least minimum compliance was achieved with 62% of participants, and full compliance with 47%. All three group sessions were attended by 54%. We note that even with this level of compliance, clinically important differences were found. Even though adherence was less than we had hoped we have got a very clear positive result on one of the trial primary outcomes. It seems likely shared decision making between patients and GPs could be important to increasing compliance, although this would need to be evaluated. For future delivery, this study demonstrated that group sessions were an important part of the intervention, The one intervention that had the least positive feedback was mindfulness. However, we are reluctant to suggest this could be removed as not all facilitators felt comfortable explaining this element of the programme.
Conclusion
Our conceptual model shows the active ingredients involved in an ideal pathway to successful tapering. This may look straightforward at first glance; however, the reality is that there are multiple contextual issues and barriers which influence someone’s ability to taper. The I-WOTCH intervention gave practical strategies, information, support, room for discussion and a tailored tapering plan to help participants navigate this difficult journey.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Yorkshire and the Humber-South Yorkshire Research Ethics Committee on 13 September 2016 (16/YH/0325). But this paper reports an embedded process evaluation. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We would like to thank the participants and intervention delivery staff who were interviewed for their valuable input into the process evaluation. Also the members of the I-WOTCH team. Alleyne Sharisse, Balasubramanian Shyam, Betteley Lauren, Booth Katie, Carnes Dawn, Furlan Andrea D, Haywood Kirstie, Iglesias Urrutia Cynthia Paola, Lall Ranjit, Manca Andrea, Mistry Dipesh, Noyes Jennifer, Rahman Anisur, Shaw Jane, Tang Nicole KY, Taylor Stephanie, Tysall Colin, Underwood Martin, Withers Emma J.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @DrHSandhu
Collaborators The I-WOTCH team: Alleyne Sharisse, Balasubramanian Shyam, Betteley Lauren, Booth Katie, Carnes Dawn, Furlan Andrea D, Haywood Kirstie, Iglesias Urrutia Cynthia Paola, Lall Ranjit, Manca Andrea, Mistry Dipesh, Noyes Jennifer, Rahman Anisur, Shaw Jane, Tang Nicole KY, Taylor Stephanie, Tysall Colin, Underwood Martin, Withers Emma J.
Contributors KS, MU, SE and HKS conceived the original design. VPN, KS and CA developed the study design and plan for data collection and undertook analysis. All authors have provided critical revisions to the manuscript and approved the final manuscript. KS is responsible for the overall content as the guarantor.
Funding This project is funded by the National Institute for Health Research (NIHR), Heath Technology Assessment (HTA) (project number 14/224/04). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA, NIHR, NHS or the Department of Health.
Competing interests MU is chief investigator or co-investigator on multiple previous and current research grants from the UK National Institute for Health Research, and Arthritis Research UK and is a co-investigator on grants funded by the Australian NHMRC and Norwegian MRC. He was an NIHR Senior Investigator until March 2021. He is a director and shareholder of Clinvivo Ltd that provides electronic data collection for health services research. He receives some salary support from University Hospitals Coventry and Warwickshire He is a co-investigator on two current and one completed NIHR funded studies that are, of have had, additional support from Stryker Ltd. Until March 2020 he was an editor of the NIHR journal series, and a member of the NIHR Journal Editors Group, for which he received a fee. KS is chief or co-investigator on many previous and current grants from the UK National Institute for Health Research. She also sat on a NIHR funding board (HS&DR) until 2018. SE is a clinician and Chief investigator on several past and ongoing trials, his department has received research support from the NIHR as well as Medtronic, Boston Scientific and Saluda Medical. He is a paid consultant to Medtronic, Saluda Medical and Mainstay Medical.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.